Abstract
Voltage-gated proton channels, HV1, trigger bioluminescence in dinoflagellates, enable calcification in coccolithophores and play multifarious roles in human health. Because the proton concentration is minuscule, exquisite selectivity for protons over other ions is critical to HV1 function. The selectivity of the open HV1 channel requires an aspartate near an arginine in the selectivity filter (SF), a narrow region that dictates proton selectivity, but the mechanism of proton selectivity is unknown. Here we use a reduced quantum model to elucidate how the Asp–Arg SF selects protons but excludes other ions. Attached to a ring scaffold, the Asp and Arg side chains formed bidentate hydrogen bonds that occlude the pore. Introducing H3O+ protonated the SF, breaking the Asp–Arg linkage and opening the conduction pathway, whereas Na+ or Cl– was trapped by the SF residue of opposite charge, leaving the linkage intact, thus preventing permeation. An Asp–Lys SF behaved like the Asp–Arg one and was experimentally verified to be proton-selective, as predicted. Hence, interacting acidic and basic residues form favorable AspH0–H2O0–Arg+ interactions with hydronium but unfavorable Asp––X–/X+–Arg+ interactions with anions/cations. This proposed mechanism may apply to other proton-selective molecules engaged in bioenergetics, homeostasis and signaling.
Similar content being viewed by others
Introduction
The voltage-gated proton channel, HV1, has been implicated in numerous biological functions in humans1: charge compensation during the respiratory burst of phagocytes killing bacteria2,3, pH homeostasis in airway epithelia4, histamine secretion by basophils5 and triggering sperm capacitation6. It is a desirable and novel drug target7 due to its involvement in various inflammatory pathologies and its exacerbation of diseases such as ischemic stroke8, breast cancer9 and chronic lymphocytic leukemia10. In other species HV1 channels play diverse roles including mediating action potentials that trigger bioluminescence in dinoflagellates11 and enabling biogenic calcite production by coccolithophores as part of the global carbon cycle12. The ability of HV1 to perform its functions would fail if its proton selectivity were not perfect, due to the low concentration of protons in biological fluids. A conserved aspartate (Asp112 in humans) in the middle of the S1 transmembrane helix is an essential part of the HV1 selectivity filter (SF)11,13. This Asp consistently interacts with the second14,15 or third16,17 Arg in the S4 segment in homology models of human HV1 (hHV1) in an open (proton-conducting) conformation. However, it is seen to interact with the second Arg in the crystal structure of a closely related voltage-sensing phosphatase in the active conformation18. Classical molecular dynamics (MD) simulations indicate that charge compensation (e.g., an intact salt bridge) appears essential19, but do not reveal the mechanism by which proton selectivity occurs. Might selectivity result from obligatory protonation and deprotonation of a titratable group13,20 lining the SF? How does an Asp in a constricted SF select protons, while rejecting other cations/anions?
Takeshita et al.21 have determined a 3.45 Å structure of a chimeric murine HV1 channel in a probable closed conformation. This structure shows that the SF Asp is located in a hydrophobic layer comprising two conserved Phe residues, which might prevent water penetration. Presumably, this hydrophobic region prevents conduction of any ions including protons in closed channels. We adopt the nearly universal assumption that channel opening involves a protein conformational change. Opening allows H3O+ to access the SF from either side of the membrane. Since no 3D structure of HV1 in an open conformation has been solved, hypotheses on proton selectivity and conduction have been based on homology models derived from the open-state structures of voltage-gated sodium or potassium channels, which share only 13–19% sequence identity with hHV122. MD simulations of hHV1 using as templates the open-state structures of the KvAP (1ORS)23 and the Kv1.2-Kv2.1 paddle chimera (2R9R)16 potassium channels predict a stable water wire in the open channel. It is widely accepted that protons can be conducted efficiently along a hydrogen-bonded water chain24,25,26,27,28. However, MD simulations of the same hHV1 channel derived from multiple templates (1ORS, 2R9R and 3RVY)14 show that the Asp–Arg interaction, which interrupts the water wire, is only occasionally broken, yielding a transient water wire. Likewise, in simulations of Ciona intestinalis HV117, which is homologous to hHV1 with 52% sequence identity, the average lifetime of a continuous water wire in an open-state model was only 6 ps. An ephemeral water-wire is suggestive of proton permeation involving titratable residues.
Whether proton selectivity could result from protonation/deprotonation of a titratable group can be answered only by considering explicit protonation/deprotonation reactions using all-electron quantum mechanical calculations, as done here. The lack of an open, proton-bound X-ray structure of hHV1 prohibits accurate evaluations of multi-ion free energy profiles for ion permeation. Thus, we evaluated selectivity by comparing the binding affinity of H3O+, Na+, Cl– and H2O in the SF, assuming that the hHV1 would be selective to the permeating ion that binds with higher affinity in the SF. A reduced SF model was devised to capture the essential chemical processes underlying proton selectivity. It was designed to maximize resemblance to the open HV1 SF and was constructed on the basis of the following considerations: At the narrowest, relatively dry region of the pore11, the SF is lined by an aspartate (Asp112 in hHV1), which is conserved in all known and putative HV11. This Asp interacts almost continuously with one of the three Arg residues in the S4 transmembrane segment in the open channel from MD simulations based on different homology models14,15,16,17,29. Even when the Asp was moved by double mutation from position 112 to 116 (D112V/V116D), it still interacted with one or two Arg residues with an intact or a broken salt-bridge in MD simulations19. Intriguingly, a positive point charge pulled through this double mutant in the broken configuration encountered a 10 kcal/mol barrier, but no barrier in the intact salt-bridge configuration19. These findings indicate that the Asp–Arg interaction is essential to proton selectivity, hence it was incorporated into the SF model. Ions such as Na+, OH– and Cl– were assumed to be dehydrated since the SF pore is purported to be narrow14,21. Ions in bulk solution were not included in the SF model, since HV1 channels are notoriously indifferent to ionic strength13, cations such as Ca2+ or Mg2+30,31, or anion species31.
To address whether proton selectivity arises from protonation and deprotonation of a titratable group, the interactions between the permeating ions and HV1 SF ligands, which play a key role in the competition between the native proton and its rivals, were treated explicitly using density functional theory to account for electronic effects such as polarization of the participating entities and differential amounts of ligand → ion charge transfer, while the region inside the SF was represented by a continuum dielectric. The proton was modeled as H3O+, while the Asp–, Arg+, Ala, His and Lys+ side chains were modeled as -CH2-COO–, -CH2-NH-C(NH2)2+, -CH2-CH3, -CH2-imidazole and -CH2-NH3+, respectively. The SF ligands were attached to a ring scaffold (see Methods) and the resulting complex was subject to all-electron geometry optimization without any constraints. The fully optimized SF geometries were then used to compute the ion-binding/exchange reactions in the HV1 pore characterized by an effective dielectric constant, ε. Since MD simulations of the open-state hHv1 model14 show that the SF is not in a bulk water environment but is relatively dry (see above), we employed ε ranging from 4 to 3032 to reflect a solvent-inaccessible or a partially solvent-exposed binding site, respectively, in order to encompass the actual value in the SF (see Methods). In interpreting results, we focus not on the absolute free energies, but on the change in ion-binding/exchange free energies with increasing ε. The approach outlined above has yielded structures and free energy trends in model SFs of various ion channels that are consistent with experimental findings32,33,34,35,36. The distance found here between the charge centers of the SF Asp and Arg (3.7 Å) agrees with that (3.8–4.6 Å) in MD simulations of the open hHV114,19. The free energy trends in the model HV1 SF found herein are also consistent with experimental findings.
Results
Binding of H3O+ in the Asp–Arg SF
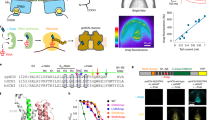
The ion-free Asp–Arg SF adopted two closed conformations that differ by <1 kcal/mol: an ion-pair conformation where the Asp and Arg side chains formed a bidentate salt bridge (Fig. 1a) and a hydrogen-bonded pair conformation where Arg protonated Asp, forming two hydrogen bonds (Fig. 1b). An Arg-carboxylate structural motif identified in several enzymes is thought to ensure rapid equilibrium between protonated and deprotonated Arg37. To see how the SF could accommodate passing ions, H3O+ was placed between Asp and Arg, above the hydrogen-bond network plane (Fig. 1c), mimicking the transient breaking of the Asp–Arg linkages, allowing H3O+ into the SF. The positioning of H3O+ between a deprotonated acid and a base has been observed spectroscopically38. In the final, fully optimized structure (Fig. 1d), the Asp and Arg side chains moved apart, breaking the two hydrogen bonds, thus opening the permeation pathway to accommodate the permeating H3O+, which transferred a proton to the SF leaving a water bridging AspH0 and Arg+. Binding of H3O+ to the Asp–Arg SF is thermodynamically favorable throughout the range of dielectric constant explored (negative ΔGx, Fig. 1e).
Binding of H3O+ to the Asp–Arg SF.
Fully optimized B3-LYP/6-31+G(3d,p) structures of (a) ion-free Asp––Arg+ SF, (b) Asp0–Arg0 SF, (c) initial configurations of the SF-H3O+ complex and (d) final configuration of the SF–H3O+ complex, AspH0–H2O–Arg+ with H in grey, C in green, N in blue and O in red. A dashed line denotes a hydrogen bond, which is defined by a donor–acceptor distance ≤3.5 Å and a H–acceptor distance ≤2.5 Å. The reaction between SF and H3O+ is depicted in (e) with free energies given in kcal/mol; ΔG1 is the binding free energy in the gas phase, whereas ΔG4 and ΔG30 are the corresponding free energies in the SF characterized by an effective dielectric constant of 4 and 30, respectively.
Binding of Cl– and Na+ to the Asp–Arg SF
The Asp–Arg SF responded quite differently to the introduction of the proton’s competitors, Cl– and Na+. We started from the “open” pore structure, where the Asp and Arg side chains were separated and placed the incoming ion between them (Fig. 2, left). Such a configuration was not favorable as during geometry optimization, the introduced ion was ejected from the pore, away from the residue bearing the same charge and became trapped by the residue carrying the opposite charge: Arg+ for Cl– and Asp– for Na+ (Fig. 2, right). In contrast to the open starting structures, the hydrogen-bond network between Asp and Arg was partially restored in the final optimized structures, closing the SF aperture and excluding other ions.
The above results highlight the importance for proton selectivity of electrostatic interactions between the SF and permeating ions. The SF Asp–Arg pair intrinsically selects protons and rejects other cations and anions: the only species that can bind favorably to both Asp– and Arg+ in an “open” state is H3O+ (Fig. 1e). Cl– and Na+ are not permeable, as they do not promote pore opening (Fig. 2).
H2O vs. H3O+ Binding in the Asp–Arg SF
Although the Asp112–Arg208 pair is broken only 10% of the time in MD simulations of a homology model of hHV1 in an open conformation, this transient disruption allows formation of a water wire that could last for 1 ns14. Would a water molecule be even more stable than H3O+ in the HV1 SF? In other words, can H3O+ displace water bound to the Asp–Arg pair? To address this question, we placed H2O in between the Asp–Arg pair and optimized the structure. The fully optimized structure in Fig. 3 (left) shows that a water molecule, unlike H3O+, cannot fully dissociate the Asp–Arg pair, as a hydrogen bond remains between the two residues. Furthermore, H3O+ can easily displace water bound to the Asp–Arg pair and protonate Asp (Fig. 3, right): The computed free energies (ΔGx, x = 1–30) for H3O+ to displace H2O from the Asp–Arg pair are all favorable (negative ΔGx, Fig. 3). The positive free energies for the reverse reaction imply that a water molecule cannot readily displace H3O+ bound to the Asp–Arg pair.
Free energies (in kcal/mol) for replacing H2O bound in Asp–Arg SF with H3O+.
See Fig. 1 legend.
The Arg208Lys Mutant is Predicted to be Proton-selective
Replacing the Lys lining the pore of voltage-gated Na+ channels with Arg nearly abolishes the channel’s selectivity for Na+ over K+39. Is Arg in the HV1 SF likewise indispensable for proton selectivity? To address this question, we replaced the SF Arg by Lys and evaluated its proton selectivity. Lys behaved like its Arg counterpart: in the ion-free state, Lys protonated Asp forming a hydrogen bond (Fig. 4a, left); however, because Lys has a lower pKa than Arg, a stable Asp––Lys+ ion pair minimum could not be found. In the ion-bound state, H3O+, which was initially placed between the protonated Asp and neutral Lys, transferred a proton to the SF leaving a water molecule to bridge AspH0 and Lys+ (Fig. 4a, right). The AspH0–H2O–Lys+ complex formation free energies remain thermodynamically favorable, although slightly less so than those for the wild-type Asp–Arg SF (compare numbers in Figs. 1e and 4a). As in the wild-type SF, during geometry optimization, Cl– and Na+ were repelled by the SF residue of the same net charge and moved towards the SF residue with the opposite charge. In the final optimized structures, Asp– and Lys+ formed a hydrogen bond, prohibiting the competing Cl– and Na+ ions from passing through the pore (Figs. 4b and 4c).
(a) Free energies (in kcal/mol) for binding of H3O+ to Lys mutant SF.
Ball and stick diagrams of the initial (left) and final (right) structures of Arg → Lys mutant SF complexes with Cl– (b) and Na+ (c). See Fig. 1 legend.
The prediction that the Lys mutant SF is selective for protons over other competing ions was verified experimentally by mutating Arg208 lining the SF to Lys: currents through the Lys208 mutant reversed near the Nernst potential for H+ (Fig. 5); the reversal potential (Vrev) did not change when Na+ or K+ replaced TMA+ or Cl– replaced CH3SO3– (Supplementary Table S5).
The Lys208 mutant is proton selective.
Measured values of Vrev at ΔpH –1.0, 0, or 1.0 (mean ± SEM, n = 3, 9, or 6, respectively), with pHo ranging 5.5 to 7.0 and pHi ranging 5.5 to 8.0. The linear regression slope was 53.3 mV/unit ΔpH, compared with the Nernst value of 58.4 mV. Inset: Proton currents in an inside out patch during pulses applied in 5 mV increments (left) indicate reversal between 0 and 5 mV (the conductance activated negative to Vrev) at pHi 7.0, with pHo 7.0 (in the pipette). Tail currents in the same patch at pHi 6.0 indicate reversal at –58 mV. Both values are near the Nernst predictions of 0 mV and –58.4 mV.
Why D112A and D112H Mutants are Chloride-selective
Mutagenesis studies13 show that replacing Asp112 in the SF with a neutral residue such as Ala or the weak base His converts the channel into an anion-selective pore. Why? To address this question we modeled two types of SF mutants: Ala0–Arg+ (Fig. 6a,b) and His0–Arg+ (Fig. 6c). Replacing anionic Asp112– with neutral Ala or His leaves the positive charge on the SF Arg+ uncompensated, which disfavors H3O+ binding to the SF due to the like charge repulsion between H3O+ and Arg+. On the other hand, strong attractive forces between the permeating OH–/Cl– and Arg+ stabilize the OH–/Cl––SF complexes and thus favor binding of the anion. To verify that the Ala112 and His112 mutants would be anion-selective, we computed the free energy for replacing H3O+ in the mutant SFs with Cl–. In line with the experimental observations, the Ala0–Arg+ SF is highly Cl–-selective in both solvent-inaccessible and exposed pores (negative ΔGx, Fig. 6a). It is predicted to be even more selective for OH– (more negative ΔGx in Fig. 6b than in Fig. 6a), in accord with the experimental finding that the Asp112Ala mutant is more permeable to OH– than to Cl–13. This is likely so because the SF Arg can protonate OH–, yielding a neutral Ala0–H2O0–Arg0 complex.
Binding of Cl– and/or OH– to H3O+-bound mutant SFs.
B3LYP/6-31+G(3d,p) fully optimized structures of H3O+–SF, Cl––SF and OH––SF complexes and free energies (in kcal/mol) for (a) [SF(Ala-Arg+)-H3O+] + Cl– → [SF(Ala-Arg+)-Cl–] + H3O+, (b) [SF(Ala-Arg+)-H3O+] + OH– → [SF(Ala-Arg+)-OH–] + H3O+ and (c) [SF(His-Arg+)-H3O+] + Cl– → [SF(His-Arg+)-Cl–] + H3O+. ΔG1 is the ion exchange free energy in the gas phase, whereas ΔG4 and ΔG30 are the corresponding free energies in the SF characterized by an effective dielectric constant of 4 and 30, respectively. If the resulting free energy is negative, the pore is Cl– or OH–-selective, but if it is positive, the pore is proton-selective.
Like the Ala0–Arg+ mutant, the His0–Arg+ SF is predicted to be also anion-selective provided the narrow pore has limited solvent accessibility (negative ΔG4), which is seen in the 3.45 Å crystal structure of a mouse HV1 chimeric channel (PDB 3WKV)21 and in simulations of open-state HV1 models14,17. However, it is predicted to be less Cl–-selective than the Ala0–Arg+ filter (less negative ΔG4 in Fig. 6c than in Fig. 6a), which is also consistent with experiment13. This is largely because H3O+ protonated the His–Arg SF, stabilizing the His+–H2O–Arg+ “reactant” complex (Fig. 6c, left), but no such stabilization can occur in the Ala0–H3O+–Arg+ “reactant” complex (Fig. 6a, left).
Discussion
Previous studies16,23 have proposed that a water wire might conduct protons through HV1, but this does not explain how other ions are excluded and why an aspartate (Asp112 in humans) in the HV1 pore is essential for proton selectivity11,13. This work shows that the HV1 Asp–Arg SF selects protons by transferring a proton from H3O+ to the SF, highlighting the importance of quantum effects (charge transfer and polarization). Although a water molecule can be inserted between Asp and Arg, it is readily displaced by H3O+ (Fig. 3), which then transfers its extra proton to the SF.
This work suggests the following proton selectivity mechanism in the HV1 SF: On a time-scale of seconds, the channel helices, S4 in particular18,40, move from a closed conformation that does not allow conduction to an open one that does. For other ion channels, opening produces a continuous water-filled pore, through which water and ions pass, often in single-file through the narrowest region41,42. For HV1, channel opening produces instead a relatively dry pore that is constricted by two hydrogen bonds formed by the SF Asp and Arg14 (Fig. 1a,b). Thermal fluctuations could transiently break the Asp–Arg linkage, allowing ions or water to approach the narrow SF (Fig. 1c, Figs. 2 and 3, left). The permeating H3O+ protonates the SF Asp, resulting in favorable AspH0–H2O0–Arg+ interactions (Fig. 1d), thus “opening” the pore to enable its own permeation, whereas anions (X–) or cations (X+) encounter unfavorable Asp––X––Arg+ or Asp––X+–Arg+ interactions and are ejected, restoring the Asp-Arg linkage (Fig. 2, right). Hence, the HV1 Asp–Arg SF intrinsically selects protons by virtue of its ability to “close” its pore when H3O+ is absent, to “open” its pore by accepting a proton when H3O+ enters, while rejecting other cations and anions though electrostatic repulsion. In the absence of permeating ions, the SF residues form hydrogen bonds that occlude the pore. Among cations, H3O+ is uniquely able to protonate the SF ligands, permeate as neutral H2O and then retrieve the excess proton (Fig. 7).
Schematic cartoon of the proposed proton selectivity mechanism by the HV1 SF.
Negatively charged Asp is red, neutral AspH0 and H2O0 are green, whereas positively charged H3O+ and Arg are light and dark blue, respectively. The dashed lines denote hydrogen bonds or salt bridges that occlude the SF pore. When H3O+ approaches the SF (left), it breaks the hydrogen bonds and protonates the SF, resulting in neutral H2O bridging AspH0 and Arg+ (middle). Transfer of a proton from the SF to H2O completes the conduction cycle (right).
The mechanism for proton selectivity found herein may also apply to other molecules. For example, if Asp112 from human HV1 is superimposed on Asp61 of the FoF1-type H+-ATPase, Arg210 aligns with Arg208 of HV1 (Fig. 8). Asp61 and Arg210 are located in the proton pathway of this H+-ATPase and are the only two amino acids that are absolutely required for function43.
A critical Asp-Arg pair in F1-Fo ATPase shares similar geometry to that in HV1.
Based on a homology model of HV1 in the open state14 and the crystal structure of F1-Fo ATPase (PDB ID 1C17), Asp112 in HV1 was superimposed onto Asp61 of F1-Fo subunit c using Chimera, which minimizes the root-mean-square deviations of superimposed atoms. This resulted in Arg208 of HV1 occupying a similar position to Arg210 of F1-Fo subunit a, which is known to participate in proton translocation.
Several other proteins, which have Asp–Arg/Lys pairs thought to be critical to proton transport, also exhibit distances between the charge centers similar to the pair in HV1. Examples of such proteins and the distances between charge centers include Na+ phosphatase, 3.9 Å44; H+ phosphatase, 4.0 Å45; and the glucose H+ symporter XylE, 4.1 Å46. In the Asp–Arg motif common to several proton pumps, a function of Arg is thought to be electrostatic ejection of the proton at the appropriate moment in the pump cycle43,47. This interacting charge pair may help enforce proton selectivity in these molecules, as in HV1.
Conversely, we searched for Asp–Arg pairs in pores of non-proton channels, where such linked acid-base pairs should not exist. We examined 60 ion channels and transporters (including various cation and anion channels, aquaporin and organic cation transporters) for which X-ray structures exist (see Supplementary Table S6). Following criteria for a proton SF established previously19, we searched for a pore-facing Asp/Glu in hydrogen-bond contact with a single Arg/Lys, located in a narrow region of the pore in an open conformation. We found no counterexample contradicting our hypothesis.
Although the interactions between ions and the known SF ligands (notably, both amino acids directly implicated in selectivity by mutation studies) have been treated in detail using all-electron quantum mechanical calculations, the contributions from other segments of the pore and ions have not been modeled explicitly in the absence of a high-resolution structure of the open-state HV1 channel. Consequently, the present results, which are in line with experimental observations, are limited to explaining proton selectivity in the constricted, relatively dry Asp-Arg SF. How the proton leaves this SF is not explicitly dealt with here. Perhaps an incoming H3O+ (or another cation) could dislodge H3O+ from the SF, as in the classical “knock-on” mechanism for K+ channels proposed by Hodgkin and Keynes42. MD simulations of the open hHV1 channel derived from multiple templates14 show that the SF is located at the extracellular end of a narrow constriction ~10 Å long with a hydrophobic region surrounding Phe150–Arg21114,15 at the inner end. Thus, another question is how protons pass through this second Phe150-Arg211 hydrophobic zone. However, in a recent computational study48, H3O+ positioned at the entrance to a hydrophobic pore was found to induce water entry, creating its own water wire and lowering the free energy barrier for proton permeation. Such a mechanism may transiently hydrate the Phe-Arg bottleneck, enabling proton hopping from one water molecule to the next. When the open HV1 channel structure becomes available, the contributions of non-SF residues, proton coupling and kinetic barriers to proton selectivity could be assessed from computed charge-transfer free energy profiles.
Methods
SF Model and Justification
Models of the hHV1 SFs were built using GaussView version 3.09, following the guidelines from our previous work32. The SF ligating groups were coordinated to the permeating ion or water and attached to a carbon–hydrogen ring scaffold via flexible methylene spacers (see Figures). The ring scaffold prevents the metal ligands from drifting away or assuming unrealistic, pore-occluding positions during geometry optimization. However, the shape and the C–H orientations of the ring do not obstruct the pore lumen. Moreover, the ion-ligating groups and their connection to the ring are flexible enough to allow them to optimize their positions upon ion/water binding.
Geometry Optimization of the SF Model
In previous studies32, the B3-LYP/6-31+G(3d,p) method was shown to be the most efficient among the various methods tested in reproducing experimentally determined molecular properties and structural characteristics of model ligands and metal complexes (see Supplementary Table S1). Hence, it was used to optimize the geometry of each model SF without any constraints and to compute the electronic energies, Eel, using the Gaussian 09 program. It was also used to compute the frequencies of each optimized structure. No imaginary frequency was found in any of the optimized structures.
Free Energy Calculations
The binding of H3O+ to a model SF to yield [H3O+-SF] is described by the following reaction

Binding of H3O+ to the wild-type or mutant HV1 SF is thermodynamically favorable only if the binding free energy for eq 1 is negative. Following Eisenman’s equilibrium theory of ion selectivity49, the filter’s selectivity can be expressed in terms of the free energy ΔGx for replacing the native H3O+ bound inside a model SF, [H3O+–SF], with a rival ligand such as water, Na+, Cl– or OH– (denoted as X)

The native H3O+ is preferred to the rival ligand X in the wild-type or mutant HV1 SF if ΔGx for eq 2 is positive or if ΔGx for the reverse reaction, [X–SF] + H3O+ → X + [H3O+–SF], is negative. Na+ or Cl– in the SF was unstable and was found near the side chain of opposite charge in the final optimized structures, precluding determination of its binding affinity.
The reaction in eq 1 or 2 was modeled to occur in vicinity of the SF so that the dielectric environment ε was assumed to be uniform for all participating entities; the respective free energy was computed using the following thermodynamic cycle:

Thus, the free energy for eq 1 or 2 can be computed as a sum of the gas-phase free energy ΔG1 and the solvation free energy ΔΔGsolvx difference between the products and reactants; i.e.,

The gas-phase free energy, ΔG1, was computed from the electronic energy (ΔEel), thermal energy (ΔEth), work term (ΔPV) and entropy differences between products and reactants,

The thermal energies including zero-point energy and entropies were computed from the B3-LYP/6-31+G(3d,p) frequencies scaled by an empirical factor of 0.961350.
The solvation free energy, ΔGsolvx, was estimated by solving Poisson’s equation with the MEAD program51 using natural bond orbital atomic charges52 and the following effective solute radii (in Å): RH = 1.50, RH(H3O+) = 1.05, RNa = 1.72, RC = 1.95, RN = 1.75, RO(H2O) = 1.85, RO(H3O+) = 1.65, RO(HO–) = 1.64, RO(COO–) = 1.56 and RCl = 2.30. The computed hydration free energies of the cations and ligands could reproduce the experimental values32,34,53 (Supplementary Table S2).
Validation against Experimental Free Energies
The methodology used to compute ΔGx has been validated against experimental ion exchange free energies between biogenic metal cations (Na+, K+ and Ca2+) in crown ethers, which resemble SF pores32 and in systems containing carboxylic ligands (nitrilotriacetic acid)34. The computed metal exchange free energies can reproduce the corresponding experimental values to within 1 kcal/mol (Supplementary Table S3)32,34,53. The methodology has yielded trends in the free energy changes that are in accord with experimental findings32,33,34,35,36,53,54,55,56. It has also yielded calculated pore aperture areas in good agreement with experimental estimates (Supplementary Table S4).
Additional Information
How to cite this article: Dudev, T. et al. Selectivity Mechanism of the Voltage-gated Proton Channel, HV1. Sci. Rep. 5, 10320; doi: 10.1038/srep10320 (2015).
References
DeCoursey, T. E. Voltage-gated proton channels: molecular biology, physiology and pathophysiology of the HV family. Physiol. Rev. 93, 599–652, doi: 10.1152/physrev.00011.2012 (2013).
DeCoursey, T. E., Morgan, D. & Cherny, V. V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422, 531–534, doi: 10.1038/nature01523 (2003).
Henderson, L. M., Chappell, J. B. & Jones, O. T. G. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246, 325–329 (1987).
Iovannisci, D., Illek, B. & Fischer, H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J. Gen. Physiol. 136, 35–46, doi: 10.1085/jgp.200910379 (2010).
Musset, B. et al. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc. Natl. Acad. Sci. USA 105, 11020–11025, doi: 10.1073/pnas.0800886105 (2008).
Lishko, P. V., Botchkina, I. L., Fedorenko, A. & Kirichok, Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337, doi: 10.1016/j.cell.2009.12.053 (2010).
Seredenina, T., Demaurex, N. & Krause, K. H. Voltage-gated proton channels as novel drug targets: From NADPH oxidase regulation to sperm biology. Antioxid. Redox Signalin press, doi: 10.1089/ars.2013.5806 (2014).
Wu, L. J. et al. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat. Neurosci. 15, 565–573, doi: 10.1038/nn.3059 (2012).
Wang, Y., Li, S. J., Wu, X., Che, Y. & Li, Q. Clinicopathological and biological significance of human voltage-gated proton channel Hv1 over-expression in breast cancer. J. Biol. Chem. 287, 13877–13888, doi: 10.1074/jbc.M112.345280 (2012).
Hondares, E. et al. Enhanced activation of an amino-terminally truncated isoform of the voltage-gated proton channel HVCN1 enriched in malignant B cells. Proc. Natl. Acad. Sci. USA 111, 18078–18083 (2014).
Smith, S. M. E. et al. Voltage-gated proton channel in a dinoflagellate. Proc. Natl. Acad. Sci. USA 108, 18162–18167, doi: 10.1073/pnas.1115405108 (2011).
Taylor, A. R., Chrachri, A., Wheeler, G., Goddard, H. & Brownlee, C. A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLoS Biol. 9, e1001085, doi: 10.1371/journal.pbio.1001085 (2011).
Musset, B. et al. Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature 480, 273–277, doi: 10.1038/nature10557 (2011).
Kulleperuma, K. et al. Construction and validation of a homology model of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 141, 445–465, doi: 10.1085/jgp.201210856 (2013).
Chamberlin, A., Qiu, F., Wang, Y., Noskov, S. Y. & Larsson, H. P. Mapping the gating and permeation pathways in the voltage-gated proton channel Hv1. J. Mol. Biol. 417, 131–145, doi: 0.1016/j.jmb.2014.11.018 (2015).
Wood, M. L. et al. Water wires in atomistic models of the Hv1 proton channel. Biochim. Biophys. Acta 1818, 286–293, doi:10.1016/j.bbamem.2011.07.045 (2012).
Chamberlin, A. et al. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. U.S.A. 111, E273–282, doi: 10.1073/pnas.1318018111 (2014).
Li, Q. et al. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 21, 244–252 (2014).
Morgan, D. et al. Peregrination of the selectivity filter delineates the pore of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 142, 625–640, doi: 10.1085/jgp.201311045 (2013).
DeCoursey, T. E. & Cherny, V. V. Voltage-activated hydrogen ion currents. J. Membr. Biol. 141, 203–223, doi: 10.1007/BF00235130 (1994).
Takeshita, K. et al. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21, 352–357, doi: 10.1038/nsmb.2783 (2014).
Pupo, A., Baez-Nieto, D., Martínez, A ., Latorre, R. & González, C. Proton channel models. Channels (Austin) 8, 180–192, doi: 10.4161/chan.28665 (2014).
Ramsey, I. S. et al. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17, 869–875, doi: 10.1038/nsmb.1826 (2010).
Eigen, M. Proton transfer, acid-base catalysis and enzymatic hydrolysis. Part I: elementary processes. Angewandte Chemie, International Edition 3, 1–19 (1964).
Myers, V. B. & Haydon, D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim. Biophys. Acta 274, 313–322, doi: 10.1016/0005-2736(72)90179-4 (1972).
Nagle, J. F. & Morowitz, H. J. Molecular mechanisms for proton transport in membranes. Proc. Natl. Acad. Sci. USA 75, 298–302 (1978).
Levitt, D. G., Elias, S. R. & Hautman, J. M. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, nonactin or valinomycin. Biochim. Biophys. Acta 512, 436–451, doi: 10.1016/0005-2736(78)90266-3 (1978).
DeCoursey, T. E. & Hosler, J. Philosophy of voltage-gated proton channels. J. R. Soc. Interface 11, 20130799, doi: 10.1098/rsif.2013.0799 (2014).
Musset, B. et al. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J. Physiol. 588, 1435–1449, doi:10.1113/jphysiol.2010.188318 (2010).
Byerly, L., Meech, R. & Moody, W., Jr. Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J. Physiol. 351, 199–216 (1984).
DeCoursey, T. E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579, doi: 10.1152/physrev.00028.2002 (2003).
Dudev, T. & Lim, C. Determinants of K+ vs. Na+ selectivity in potassium channels. J. Am. Chem. Soc. 131, 8092–8101 (2009).
Dudev, T. & Lim, C. Factors governing the Na+ vs. K+ selectivity in sodium ion channels. J. Am. Chem. Soc. 132, 2321–2332 (2010).
Dudev, T. & Lim, C. Competition among Ca2+, Mg2+ and Na+ for ion channel selectivity filters: Determinants of metal ion selectivity. J. Phys. Chem. B 116, 10703–10714 (2012).
Dudev, T. & Lim, C. Importance of metal hydration on the selectivity of Mg2+ vs. Ca2+ in magnesium ion channels. J. Am. Chem. Soc. 135, 17200−17208, doi: 10.1021/ja4087769 (2013).
Dudev, T. & Lim, C. Evolution of eukaryotic ion channels: Principles underlying the conversion of Ca2+-Selective to Na+-selective channels. J. Am. Chem. Soc. 136, 3553−3559, doi: 10.1021/ja4087769 (2014).
Guillén Schlippe, Y. V. & Hedstrom, L. A twisted base? The role of arginine in enzyme-catalyzed proton abstractions. Arch. Biochem. Biophys. 433, 266–278 (2005).
Mohammed, O. F., Pines, D., Dreyer, J., Pines, E. & Nibbering, E. T. Sequential proton transfer through water bridges in acid-base reactions. Science 310, 83–86, doi: 10.1126/science.1117756 (2005).
Favre, I., Moczydlowski, E. & Schild, L. On the structural basis for ionic selectivity among Na, K and Ca in the voltage-gated sodium channel. Biophys. J. 71, 3110–3125 (1996).
Gonzalez, C., Rebolledo, S., Perez, M. E. & Larsson, H. P. Molecular mechanism of voltage sensing in voltage-gated proton channels. J. Gen. Physiol. 141, 275–285, doi:10.1085/jgp.201210857 (2013).
Dani, J. A. & Levitt, D. G. Water transport and ion-water interaction in the gramicidin channel. Biophys. J. 35, 501–508 (1981).
Hodgkin, A. L. & Keynes, R. D. The potassium permeability of a giant nerve fibre. J. Physiol. 128, 61–88 (1955).
Miller, M. J., Oldenburg, M. & Fillingame, R. H. The essential carboxyl group in subunit c of the F1F0 ATP synthase can be moved and H+-translocating function retained. Proc. Natl. Acad. Sci. USA 87, 4900–4904 (1990).
Luoto, H. H., Nordbo, E., Baykov, A. A., Lahti, R. & Malinen, A. M. Membrane Na+-pyrophosphatases can transport protons at low sodium concentrations. J. Biol. Chem. 288, 35489–35499, doi:doi: 10.1074/jbc.M113.510909 (2013).
Lin, S. M. et al. Crystal structure of a membrane-embedded H+-translocating pyrophosphatase. Nature 484, 399–403 (2012).
Sun, L. et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490, 361–366 (2012).
Buch-Pedersen, M. J., Pedersen, B. P., Veierskov, B., Nissen, P. & Palmgren, M. G. Protons and how they are transported by proton pumps. Pflügers Arch. 457, 573–579 (2009).
Peng, Y., Swanson, J. M., Kang, S.-g., Zhou, R. & Voth, G. A. Hydrated excess protons can create their own water wires. J. Phys. Chem. B, doi:10.1021/jp5095118 (2014).
Eisenman, G. in Symposium on Membrane Transport and Metabolism (eds A. Kleinzeller & A. Kotyk ) 163–179 (Academic Press 1961).
Wong, M. W. Vibrational frequency prediction using density functional theory. Chem. Phys. Lett. 256, 391–399 (1996).
Bashford, D. in Scientific Computing in Object-Oriented Parallel Environments Vol. 1343 Lecture Notes in Computer Science (eds Yutaka Ishikawa, RodneyR Oldehoeft, JohnV W. Reynders, & Marydell Tholburn ) Ch. 30, 233–240 (springer Berlin Heidelberg 1997).
Reed, A., Weinstock, R. & Weinhold, F. Natural population analysis. J. Chem. Phys. 83, 735–746 (1985).
Dudev, T. & Lim, C. Why voltage-gated Ca2+ and bacterial Na+ channels with the same EEEE motif in their selectivity filters confer opposite metal selectivity. Phys. Chem. Chem. Phys. 14, 12451–12456, doi: 10.1039/C2CP00036A (2012).
Dudev, T. & Lim, C. Bidentate vs. monodentate carboxylate coordination modes in magnesium and calcium proteins: What are the basic principles? J. Phys. Chem. B 108, 4546–4557 (2004).
Dudev, T., Chang, L.-Y. & Lim, C. Factors governing the substitution of La3+ for Ca2+ and Mg2+ in metalloproteins: A DFT/CDM study. J. Am. Chem. Soc. 127, 4091–4103 (2005).
Dudev, T. & Lim, C. Competition between Li+ and Mg2+ in Metalloproteins. Implications for Lithium Therapy. J. Am. Chem. Soc. 133, 9506–9515 (2011).
Acknowledgements
This work was supported by Academia Sinica, MOST, Taiwan (Grant NSC-98- 2113-M-001-011), NSF MCB-1242985 (T.E.D. & S.M.E.S.) and NIH: GM102336 (T.E.D. & S.M.E.S.). T.D. is supported by the Institute of Biomedical Sciences at Academia Sinica and EU Grant “Beyond Everest”, FP7-REGPOT-2011-1.
Author information
Authors and Affiliations
Contributions
T.D. performed the calculations. B.M., D.M. and V.C. conducted patch-clamp studies and analyzed results. S.M.E.S. provided constructs. S.M.E.S. and K.M. performed PDB data analysis. T.D., S.M.E.S. and K.M. prepared figures, T.E.D. and C.L. designed the project and discussed results. T.D., T.E.D. and C.L. participated in writing the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Dudev, T., Musset, B., Morgan, D. et al. Selectivity Mechanism of the Voltage-gated Proton Channel, HV1. Sci Rep 5, 10320 (2015). https://doi.org/10.1038/srep10320
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10320
This article is cited by
-
Voltage-gated proton channels from fungi highlight role of peripheral regions in channel activation
Communications Biology (2021)
-
Molecular determinants of proton selectivity and gating in the red-light activated channelrhodopsin Chrimson
Scientific Reports (2017)
-
How the extra methylene group affects the ligation properties of Glu vs. Asp and Gln vs. Asn amino acids: a DFT/PCM study
Journal of Molecular Modeling (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.