Abstract
The efficient removal of trace carcinogenic organic pollutants, such as polycyclic aromatic hydrocarbons (PAHs) and ionic dyes, from water is an important technical challenge. We report a highly effective recyclable multifunctional azobenzene (AZ)-based silica-supported polymeric adsorbent which can simultaneously remove both PAHs and anionic dyes from water to below parts per billion (ppb) level based on multiple interactions such as the hydrophobic effect, π–π stacking and electrostatic interactions, thus providing a new strategy for designer water remediation materials.
Similar content being viewed by others
Introduction
PAHs are toxic, mutagenic, carcinogenic and are among the most damaging pollutants with regard to the ecosystem1,2,3,4. Although they have low water solubility, PAHs are strongly bioaccumulative5,6 (e.g. by fish) and can thus pass up the food chain to top predators, including man. However, traditional low cost adsorbents such as activated carbon have weak affinity for PAH (K ~ 105 M−1, which corresponds to 2000 ppb of residual PAH in water)7. Thus they are only suitable for preliminary water clean-up on a large scale. More stringent water processing requires much more effective adsorbents than activated carbon in order to significantly reduce the level of residual PAH in water because in most cases, the residual PAH concentration in water is less than 2000 ppb. Generally, organic hosts are more effective than inorganic hosts in this context, because the former can undergo conformational rearrangement to match the PAH molecular structure, whereas inorganic hosts are typically rather more rigid. Several organic materials have been reported to capture PAHs8,9,10,11,12, with the most efficient poly(propylene imine) dendrimers being capable of reducing the concentration of aqueous pyrene, a representative PAH, to several ppb11. On the other hand, hydrophilic organic dyes are ubiquitous water pollutants that are both toxic to humans and known to adversely affect aquatic ecosystems while being difficult to directly remove from water. Therefore, dendritic polymers and their derivatives have been developed to transfer hydrophilic dyes from water into oil13,14,15,16,17,18,19. However, an oil–water biphasic system is a sub-optimal choice for water treatment because of secondary pollution. The problem of effective water remediation has encouraged chemists and materials scientists to develop next-generation adsorbents using new design principles. For example, recently we prepared a homopolymer vesicle for facile immobilization of gold nanoparticles and effective catalysis, as well as pyrene adsorption by π–π interactions with the naphthalenyl groups in the vesicle membrane20. This material shows efficient adsorption capacity for hydrophobic PAHs based on π–π interactions. However, multifunctional adsorbents need to be designed based on multiple interactions to meet various requirements such as simultaneous adsorption of both hydrophobic and hydrophilic dyes, easy separation and regeneration, etc.
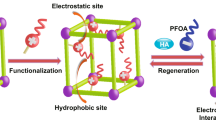
Herein we describe the design and preparation of a new recyclable multifunctional silica-supported AZ-based polymeric adsorbent (Figure 1) that can simultaneously remove both PAHs and hydrophilic dyes from water with high efficiency (e.g. to below ppb scale for typical PAHs) based on multiple interactions with PAHs such as π–π stacking of azobenzene group, hydrophobic effect and electrostatic interactions in polluted water21. Firstly, the azobenzene (AZ) derivative which was derived from 4-phenyldiazenylphenol to 2-[4-phenyldiazenylphenyloxy]ethoxy-ethanol (AZ-OH) and then to {4-[2-(2-oxiraneylmethoxy-ethoxyl)-phenyl}-phenyl-diazene (Figures S1–S3) is designed to enhance the PAH removal efficiency via π–π stacking interactions and the hydrophobic effect between the azobenzene units and PAHs. Secondly, a multicompartment structure22,23,24 should facilitate capture of a range of various guest pollutants and the hydrophilic poly(ethylene glycol) (PEG) component should enhance pollutant capture. Finally, the cationic branched polyethylenimine (PEI) is selected so as to not only bridge the silica support and the AZ and PEG components, but also to adsorb hydrophilic anionic dyes via electrostatic interaction and van de Waals forces8,9,10. The silica in the core of the adsorbent was incorporated to aid the recycling of the adsorbent. Our strategy offers the following advantages in water remediation: (1) highly efficient removal of pollutants (the residual PAH concentration is at the sub-ppb level); (2) simultaneous removal of different organic pollutants from polluted water using just one adsorbent; (3) facile recycling of the adsorbent; (4) due to the easy separation, this adsorbent can be used to produce high-quality drinking water while minimizing mankind's exposure to PAHs; (5) the azobenzene strategy can be extended to design a range of new-generation adsorbents for removing PAHs.
A multifunctional azobenzene (AZ)-based polymeric adsorbent SiO2@PEI@AZ/PEG on the basis of multiple interactions for effective simultaneous removal of trace carcinogenic polycyclic aromatic hydrocarbons (PAHs) and anionic dyes in water.
(i) Acid-catalyzed silanol condensation; (ii) and (iii) oxirane-amine coupling (PEI has 232 repeat units on average but only several are shown here for clarity). The polycyclic aromatic hydrocarbons (PAHs) in the polluted water can be removed based on the hydrophobic effect and π–π stacking with AZ moieties, whereas the anionic dyes can be simultaneously removed by electrostatic interaction and van de Waals forces with PEI. The collected pollutants can be easily removed by washing with chloroform/aqueous NaOH solution and thus the adsorbent is recycled.
Results
Synthesis of functional silica particles
Silica particles with an average diameter of 200 μm are activated using aqueous HCl (the pore size of 3.3 nm and the surface area of 437 m2/g remain almost intact by BET surface area measurements before and after the activation, see Figure S4), dispersed in toluene and treated with 3-glycidyloxypropyltrimethoxysilane (GPTMS) in the presence of triethylamine (the pore size was reduced to <2 nm and the surface area to 308.2 m2/g). Glycidyl (epoxy) groups are thus introduced onto the silica surface via silanol condensation, leading to an increase in mass of 18.5 wt.% (partly due to the silane component) as judged by thermogravimetric analysis (TGA, Figure S5).
Synthesis of SiO2@PEI
PEI (232 repeat units) can then be attached to the silica particles (leading to a further 4.7 wt.% mass increase by TGA) via epoxy-amine coupling. The resulting material has a significantly lower porosity, with the surface area reduced to 2.9 m2 g−1. Since PEI has a typical diameter of 4 nm, while the silica precursor has a pore size of less than 2 nm, it is unlikely that PEI enters the pore. Therefore, the decrease of surface area most likely results from the reaction of PEI at the mouth of the pore rather than entering into the pore and the PEI has been only attached onto the outer surface of the silica.
Synthesis of SiO2@PEI@AZ/PEG
A large fraction of the primary and secondary amines on the PEI chains remain unreacted and some of these are subsequently coupled with epoxy derivatives of azobenzene (AZ) and poly(ethylene glycol) (PEG) (2.6 wt.% mass increase as judged by TGA). To further analyze the organic composition in the SiO2@PEI@AZ/PEG adsorbent, the silica core was removed by treatment with aqueous hydrogen fluoride (HF). The organic component was isolated and confirmed to have a mean composition of PEI-AZ16-PEG38 by 1H NMR spectroscopy (Figure S6) and elemental microanalyses (see supporting information). Thus on average 38 PEG chains and 16 AZ derivatives are attached to one branched PEI chain. For comparison, adsorbents without the silica support such as PEI@AZ/PEG (PEI-AZ19-PEG46), PEI@PEG (PEI-PEG90)25 and PEI@AZ (PEI-AZ48) were also synthesized (Figures S7-S8) and evaluated in terms of their solution behavior and PAH affinity, as discussed below. Moreover, SiO2@PEI-AZ3-PEG40, SiO2@PEI-AZ33-PEG40 and SiO2@PEI-PEG40 were also synthesized for determining the effect of AZ content on the efficiency of PAH cleanup.
Water remediation
The multifunctional SiO2@PEI@AZ/PEG adsorbent was expected to efficiently remove pyrene from water based on the three interactions indicated in Figure 1. To test this hypothesis, the SiO2@PEI@AZ/PEG adsorbent was added to pyrene-contaminated water and allowed to stand for a given time period (1–3 days). To determine the concentration of residual pyrene in the treated water by fluorescence spectroscopy, the adsorbent was isolated by filtration.
Discussion
Each component in the adsorbent particle plays a unique role. The control experiments showed that the AZ component was essential to the efficient cleanup of pyrene. For example, in the case of SiO2@PEI or SiO2@PEI@PEG, the residual pyrene in water could not be reduced to below 6.5 and 6.3 ppb, respectively. On the other hand, PEG was necessary to speed up the adsorption. It was found that for a control adsorbent of SiO2@PEI@AZ (containing no PEG), approximately 48 h was required to attain adsorption equilibrium (Figure 2A). In contrast, it took about 14 h for the SiO2@PEI@AZ/PEG adsorbent to reach equilibration, indicating that the PEG chains enhance the rate of PAH clean-up. This PEG acceleration effect may be the result of microphase separation of the organic host in water. In the case of SiO2@PEI@AZ, the AZ domain tends to form a coating layer whereas in the case of SiO2@PEI@AZ/PEG the hydrophobic AZs are separated by the surrounding PEG chains, forming a multi-compartment structure (the mechanism will be further discussed below), leading to a faster PAH adsorption rate.
Fluorescence intensities of water samples: (A) PEG in SiO2@PEI@AZ/PEG adsorbent can speed up the adsorption of pyrene; (B) Residual fluorescence intensities of water samples after adsorption with different doses of SiO2@PEI@AZ/PEG; The pyrene clean-up efficiency is not influenced either by acidic conditions (pH 5.4, acetate buffer) (C) or by anionic rose bengal (D).
Conditions: [pyrene] = 137 ppb; adsorption for 3 d in buffer unless stated otherwise; λex = 335 nm. The measurement errors at very low pyrene concentration (ca. 0.1 ppb) are within 50%.
It has previously been reported that branched PEI alone can reduce the aqueous pyrene concentration to 1–30 ppb8,9,10. However, for the SiO2@PEI@AZ/PEG adsorbent, the hydrophobic effect and π–π stacking effect should enhance the adsorption capacity and reduce the residual PAH to a much lower level than that achieved for branched PEI alone. To optimize the minimum loading required for efficient pyrene removal, various SiO2@PEI@AZ/PEG adsorbent doses were mixed with polluted water and the residual pyrene levels determined via fluorescence spectroscopy (Figure 2B). It was confirmed that a dose of 0.215 g L−1 of SiO2@PEI@AZ/PEG adsorbent (~eq. 9 AZs to 1 pyrene or 6.2 × 10−5 g PAH per gram of adsorbent) was sufficient for pyrene clean-up, leading to a residual pyrene concentration of 0.2 ± 0.1 ppb in water (higher doses did not lead to better performance). The residual pyrene concentration was determined from a calibration curve (Figure S9) and a reference sample. The adsorption profile (Figure S10) appears to comply with a Langmuir model, i.e., a monolayer adsorption mechanism. However, the adsorption constant K is so large that the measurement error does not allow precise determination of the K value. However, K can be determined from a literature method12 (where K = 1/[PAH]) and is in the range of 4 × 108 to 1 × 109 L mol−1. As far as we are aware, this is the highest value reported to date.
In order to further verify the role of AZ component in the SiO2@PEI@AZ/PEG adsorbent, water remediation was conducted under acidic conditions; as pyrene release should occur below pH 5.4 if PEI alone is responsible for the pyrene capture9,10. As shown in Figure 2C, the residual pyrene concentration is ~0.2 ppb, suggesting that the acidic conditions exert no influence on the ability of the SiO2@PEI@AZ/PEG adsorbent to capture trace pyrene in water. Since SiO2 has only a very weak affinity for pyrene26, we conclude that the AZ domains in the SiO2@PEI@AZ/PEG adsorbent are responsible for pyrene capture, rather than the PEI component.
In another experiment, the SiO2@PEI@AZ/PEG adsorbent was fully saturated with anionic rose bengal prior to pyrene exposure. As expected, the pyrene was just as efficiently captured as in the absence of any rose bengal (Figure 2D). Since rose bengal resides in the PEI domain due to strong electrostatic interactions and thereby expel any pyrene in the PEI domain27, this observation suggests that the PEI and AZ components are located in different domains and each mediates the capture of different guest species. Moreover, inorganic ions exert no influence on pyrene capture, because the pyrene clean-up efficiency remains essentially the same regardless of the buffer concentration from 0.004 to 0.10 M (spectra not shown). Other PAHs, such as perylene and 3,4-benzopyrene, were also evaluated, with good clean-up efficiencies being achieved in both cases (<0.2 ppb for both PAHs, Table 1).
It was found that the AZ content has to be above a certain value (16 AZs per PEI) to ensure efficient PAH cleanup. Both SiO2@PEI-PEG40 and SiO2@PEI-AZ3-PEG40 exhibited very low cleanup efficiency, whereas SiO2@PEI-AZ16-PEG38 was as efficient as SiO2@PEI-PEG40, suggesting that the formation of AZ domains is necessary for efficient PAH cleanup.
To reveal the advantages of our multifunctional silica-supported polymeric SiO2@PEI@AZ/PEG adsorbent, another reference adsorbent, PEI@AZ, was also evaluated. Unfortunately, PEI@AZ is insoluble in water and can only very slowly remove pyrene (>2 days) due to its limited surface area. This indicates that hydrophilic components such as PEG and SiO2 are essential for good performance.
To further probe the remediation mechanism, the organic component of the PEI@AZ/PEG was independently synthesized and evaluated with respect to its self-assembly behavior. PEI@AZ/PEG is readily dispersible in solvents such as chloroform and water. When the yellow PEI@AZ/PEG is dispersed in chloroform, a clear system forms and the PEI@AZ/PEG cannot be extracted into water. Atomic force microscopy (AFM; Figure 3) studies suggest that PEI@AZ/PEG exists as rather uniform small particles (typical width × height = 90 nm × 8 nm). Interestingly, if PEI@AZ/PEG is first dispersed in water, it cannot be extracted into chloroform. Transmission electron microscopy (TEM) studies show that the nanoparticles formed in water have a number-average diameter of 120 nm. A core-shell structure with multi-compartment domains in the core (Figure 3) can be clearly visualized. Therefore, it is likely that the organic component in the SiO2@PEI@AZ/PEG adsorbent has a strong propensity to form multi-compartment structures. This is further supported by 1H NMR analysis of PEI@AZ/PEG in chloroform and water, respectively (Figure S8). The signal intensity of the AZ component relative to that of all the other protons is reduced by 25% in water compared to that in chloroform, indicating that the AZ groups are less solvated in water. Further UV/visible spectroscopy studies of PEI@AZ/PEG in water reveal a red shift compared with that of AZ-OH (Figure 4A), which indicates π–π stacking of the AZ groups28. This medium-dependent self-assembly behavior facilitates recycling of the adsorbent. For example, it was confirmed that when the SiO2@PEI@AZ/PEG adsorbent was dried and washed with chloroform, the adsorbed PAH molecules were completely released. UV/vis analysis suggests that the AZ groups in chloroform no longer undergo π–π stacking, as compared to that of the AZ-OH small molecule (Figure 4B). Moreover, when treated with aqueous NaOH for several days, the sequestered anionic dyes were mostly washed away, too. The recycled SiO2@PEI@AZ/PEG adsorbent shows similar PAH and dye removal efficiency to a fresh sample, i.e., 6.2 × 10−5 g PAH per gram of adsorbent and 0.8 wt.% for dyes.
PEI@AZ/PEG (no silica support) in different media forms different assemblies.
(A) PEI@AZ/PEG initially dropped into water can no longer be extracted into chloroform and vice versa; (B) AFM micrograph of PEI@AZ/PEG in chloroform after solvent evaporation; (C) TEM micrograph of aqueous PEI@AZ/PEG after water evaporation. The core-shell structure of a multi-compartment micelle is clear. Conditions: [PEI@AZ/PEG] = 10−6 M in water or chloroform.
The UV/vis spectra of (A) PEI@AZ/PEG in water shows a red shift compared with that of AZ-OH, suggesting π–π stacking of the AZ groups in water; and (B) PEI@AZ/PEG and AZ-OH in chloroform, where PEI@AZ/PEG shows no red shift when compared to AZ-OH, indicating no π–π stacking of AZ groups in chloroform.
The affinity of the AZ domains towards PAHs (most likely due to π–π stacking interactions) is confirmed by a fluorescence-quenching experiment. When the PEI@AZ/PEG concentration is increased to 1.5 × 10−5 M, fluorescence of pyrene is completely quenched (Figure 5). This indicates that pyrene is captured within the PEI@AZ/PEG nanoparticles because fluorescent quenching can only occur when the donor and acceptor molecules are in close proximity. The AZ component is confirmed to be the quencher because, when PEI@PEG is used in place of PEI@AZ/PEG, no pyrene quenching is observed. From the viewpoint of Förster resonance energy transfer (FRET), the AZ absorbance at 310–410 nm partly overlaps with the pyrene emission at 360–450 nm, thus constituting a donor/acceptor FRET system. Inspecting Figure 2, the pyrene appears to reside exclusively in the AZ domains. Although it was reported that polyamines exhibit high binding affinity towards pyrene (K ~ 105 L mol−1)10, our observations (K ~ 4 × 108 ~ 1 × 109 L mol−1) show that the AZ domains have a much higher binding affinity for pyrene than PEI, presumably due to the hydrophobic effect and π–π stacking interactions.
More importantly, the PEI@AZ/PEG formulation is highly selective towards PAH capture. At 1.5 × 10−5 M, it quenches aqueous fluorescence due to 137 ppb pyrene (Figure 5). Moreover, no fluorescence is recovered on addition of 1-bromodecane, benzene or toluene (Figure S11), indicating that these hydrophobic competitor molecules cannot displace the pyrene from the AZ domains. It has been reported that surfactant micelles can solubilize PAHs28. However, the addition of anionic surfactants such as SDS (concentration > critical micelle concentration (CMC)) to the adsorbent solution has little effect on the pyrene fluorescence, as the anionic surfactant is likely to be adsorbed by the cationic PEI. In case of cationic surfactants such as cetyltrimethylammonium chloride (concentration > CMC), the fluorescence of pyrene can only be partly recovered, which confirms the adsorbent's high affinity for PAHs. Similar performance is achieved using the SiO2@PEI@AZ/PEG adsorbent (Figure 6), also confirming its high selectivity towards PAHs.
Excellent adsorption selectivity of SiO2@PEI@AZ/PEG: Fluorescence of aqueous pyrene after adsorption with SiO2@PEI@AZ/PEG in the absence/presence of bromodecane, toluene, benzene, sodium dodecyl sulfate (SDS).
Conditions: pH 7.4 (buffer); λex = 335 nm; [pyrene] = 137 ppb; [PEI@ AZ/PEG] = 1.5 × 10−5 M; [SDS] = 5 × 10−4 M; equilibrating for 24 h before fluorescence measurement; the fluorescence of neat pyrene alone is also presented for reference.
Because of the high cationic charge density of PEI, anionic dyes in water are efficiently removed using the SiO2@PEI@AZ/PEG adsorbent. Various anionic dyes (Chart S1) were evaluated and in each case decoloration occurs (Figure S12). To precisely determine the residual concentration of the dyes in water, a bulk sample is concentrated by 10–1000 times prior to UV/visible spectroscopy studies (Table 2). Using this technique, the concentration of residual dyes in water is at the range of 10−8 ~ 10−9 M. The residual concentration of rose Bengal, erythrosine B and eosin Y are readily detected using fluorescence spectroscopy and were found to be around 10−9 M, or lower (Figures S13 and S14). The dye absorption capacity is approximately 0.8 wt.% with respect to the SiO2@PEI@AZ/PEG adsorbent, which corresponds to 2–5 dye molecules per PEI. The adsorption should follow a Langmuir model (Figure S10B). Thus the SiO2@PEI@AZ/PEG adsorbent allows efficient removal of large water-soluble anionic dyes. Moreover, both PAHs and anionic dyes can be simultaneously removed from water with high efficiency.
To demonstrate the practicality and real-world application of the SiO2@PEI@AZ/PEG adsorbent, a glass column (Figure 7) was filled with the adsorbent. The pyrene-contaminated water was added from top of the column and flowed through the regenerated adsorbent (Rhodamine B was added into water for better demonstration in this figure). The water remains at a height of 50 mm. Under this slight gravity, the purified water flow flux is 132 kg/(min·m2) and the pyrene can be removed to a residual pyrene concentration of 0.2–0.3 ppb. The flow flux can be further increased by using the pressure from a tap. Therefore, this adsorbent may be used for drinking water purification at home. Alternatively, it may be filled in a portable water purification apparatus for emergency.
In conclusion, we have successfully prepared a highly effective multifunctional recyclable AZ-based silica-supported polymeric adsorbent SiO2@PEI@AZ/PEG. The AZ groups and PEG chains are randomly conjugated to the PEI shell and undergo microphase separation to form a hydrophobic AZ-rich domain and a hydrophilic PEI domain. The AZ domain shows strong affinity towards PAHs due to π–π stacking interactions and can reduce the aqueous concentration of PAHs such as pyrene to below 0.5 ppb. The PEI domain can independently remove anionic organic dyes with high efficiency due to complementary electrostatic interactions. This multifunctional material allows facile separation, high clean-up efficiency and recyclability; it can be used to produce high-quality drinking water while minimizing mankind's exposure to PAHs via the food chain. Moreover, this strategy can be extended to design a range of new adsorbents for removing PAHs and heavy metals from water.
Methods
Synthesis of SiO2@PEI@AZ and etching of the silica
The synthesis was similar to that of SiO2@PEI@AZ/PEG replacing epoxy PEG with an equivalent number of moles of epoxy AZ. A mixture of SiO2@PEI (0.8 g) and epoxy AZ (0.33 g) in chloroform (10 mL) was prepared and stirred at 60°C for 3 days. The solid was separated and fully washed with fresh ethanol before drying. Yielded 0.92 g of red solid. The etching of silica was carried out in a similar method to that described for SiO2@PEI@AZ/PEG29.
Water remediation
Typically, to a stock solution of pyrene-contaminated (137 ppb) and buffered water (phosphate, 0.01 M), solid adsorbent SiO2@PEI@AZ/PEG (5.0 mg) was added with slight shaking, followed by standing for a desired time. After removal of the solid adsorbent by centrifugation or filtration, the water was subjected to fluorescent detection. The λex was set at 335 nm and the emission within 350–550 nm was recorded. The residual concentration of pyrene could be determined from a fluorescent intensity-concentration calibration curve.
For a calibration curve, a stock solution of pyrene was diluted with fresh buffered water while the fluorescent intensity was monitored under similar measurement conditions.
Because the fluorescence intensities of perylene and 3,4-benzopyrene in water are weak, the calibration curves are established in chloroform. A treated water sample is extracted with chloroform and concentrated prior to fluorescence detection.
Simultaneous loading of PAH and hydrophilic dyes in SiO2@PEI@AZ/PEG
Typically, solid adsorbent SiO2@PEI@AZ/PEG (2.0 mg) was fully saturated with anionic dye by dropping into an aqueous solution of rose Bengal (10 mL, 1 × 10−4 M) while shaking for 24 h, then the solid adsorbent (became red because of the captured rose Bengal) was separated and washed with fresh buffered water (pH 7.4) before being dropped into a pyrene-contaminated stock water (4 mL, 137 ppb). The mixture was allowed to stand for 24 h. After removal of the solid adsorbent, the water was subjected to fluorescent detection to determine the residual pyrene.
The simultaneous removal of PAH and ionic dye was also carried out. Typically, solid adsorbent SiO2@PEI@AZ/PEG (5.0 mg) was dropped into a buffered aqueous solution (10 mL, pH 7.4) containing rose Bengal (1 × 10−6 M) and pyrene (137 ppb). The mixture was allowed to stand for 3 day. After removal of the solid adsorbent, the water was subjected to fluorescent detection to determine the residual pyrene and rose Bengal.
Reusability of the adsorbent
To remove the PAH captured in the adsorbent SiO2@PEI@AZ/PEG, the adsorbent separated from polluted water is washed with ethanol to remove the water (or dried under vacuum), then washed with chloroform, followed by drying.
To remove both dyes and PAHs, the adsorbent is first submerged in aqueous NaOH (pH > 11) for 4 h and filtered; this process is repeated three times and then washed with fresh water, ethanol and chloroform in sequence.
References
Mahanty, B., Pakshirajan, K. & Dasu, V. V. Understanding the Complexity and Strategic Evolution in PAH Remediation Research. Crit. Rev. Env. Sci. Tec. 41, 1697–1746 (2011).
Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ. Chem. Lett. 5, 169-195 (2007).
Troisi, G. M., Bexton, S. & Robinson, I. Polyaromatic hydrocarbon and PAH metabolite burdens in oiled Common Guillemots (Uria aalge) stranded on the East Coast of England (2001–2002). Environ. Sci. Technol. 40, 7938–7943 (2006).
White, K. L. An overview of immunotoxicology and carcinogenic polycyclic aromatic hydrocarbons. Environ. Carcinogenesis Rev. 4, 163–202 (1986).
Beyer, J., Jonsson, G., Porte, C., Krahn, M. M. & Ariese, F. Analytical methods for determining metabolites of polycyclic aromatic hydrocarbon (PAH) pollutants in fish bile: A review. Environ. Toxicol. Phar. 30, 224–244 (2010).
van der Oost, R., Beyer, J. & Vermeulen, N. P. E. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Phar. 13, 57–149 (2003).
Li, D. & Ma, M. Nanoporous polymers: New nanosponge absorbent media. Filtr. Sep. 36, 26–28 (1999).
Arkas, M., Eleades, L., Paleos, C. M. & Tsiourvas, D. Alkylated hyperbranched polymers as molecular nanosponges for the purification of water from polycyclic aromatic hydrocarbons. J. Appl. Polym. Sci. 97, 2299–2305 (2005).
Kannaiyan, D. & Imae, T. pH-dependent encapsulation of pyrene in PPI-core: PAMAM-Shell dendrimers. Langmuir 25, 5282–5285 (2009).
Pistolis, G., Malliaris, A., Tsiourvas, D. & Paleos, C. M. Poly(propyleneimine) dendrimers as pH-sensitive controlled-release systems. Chem-Eur. J. 5, 1440–1444 (1999).
Arkas, M., Tsiourvas, D. & Paleos, C. M. Functional dendrimeric “nanosponges” for the removal of polycyclic aromatic hydrocarbons from water. Chem. Mater. 15, 2844–2847 (2003).
Xie, S. M., Zhang, M., Wang, Z. Y. & Yuan, L. M. Porous metal membranes for solid-phase extraction of polycyclic aromatic hydrocarbons. Analyst 136, 3988–3996 (2011).
Baars, M. W. P. L., Froehling, P. E. & Meijer, E. W. Liquid-liquid extractions using poly(propylene imine) dendrimers with an apolar periphery. Chem. Commun. 1959–1960 (1997).
Stiriba, S. E., Kautz, H. & Frey, H. Hyperbranched molecular nanocapsules: Comparison of the hyperbranched architecture with the perfect linear analogue. J. Am. Chem. Soc. 124, 9698–9699 (2002).
Wan, D. C., Pu, H. T. & Cai, X. Y. Separation promoted by molecular recognition of a core engineered macromolecular nanocapsule. Macromolecules 41, 7787–7789 (2008).
Wan, D. C., Wang, G. C., Pu, H. T. & Jin, M. Can nonspecific host-guest interaction lead to highly specific encapsulation by a supramolecular nanocapsule? Macromolecules 42, 6448–6456 (2009).
Wang, R., Yu, B., Jiang, X. S. & Yin, J. Understanding the host-guest interaction between responsive core-crosslinked hybrid nanoparticles of hyperbranched poly(ether amine) and dyes: the selective adsorption and smart separation of dyes in water. Adv. Funct. Mater. 22, 2606–2616 (2012).
Wilms, D., Stiriba, S. E. & Frey, H. Hyperbranched Polyglycerols: From the Controlled Synthesis of Biocompatible Polyether Polyols to Multipurpose Applications. Acc. Chem. Res. 43, 129–141 (2010).
Zhou, Y. F., Huang, W., Liu, J. Y., Zhu, X. Y. & Yan, D. Y. Self-assembly of hyperbranched polymers and its biomedical applications. Adv. Mater. 22, 4567–4590 (2010).
Zhu, Y. Q., Fan, L., Yang, B. & Du, J. Z. Multifunctional homopolymer vesicles for facile immobilization of gold nanoparticles and effective water remediation. ACS Nano 8, 5022–5031 (2014).
Wright, A. T. & Anslyn, E. V. Differential receptor arrays and assays for solution-based molecular recognition. Chem. Soc. Rev. 35, 14–28 (2006).
Du, J. Z. & Chen, Y. M. Organic-inorganic hybrid nanoparticles with a complex hollow structure. Angew. Chem., Int. Ed. 43, 5084–5087 (2004).
Du, J. Z. & Armes, S. P. Patchy multi-compartment micelles are formed by direct dissolution of an ABC triblock copolymer in water. Soft Matter 6, 4851–4857 (2010).
Du, J. Z. & O'Reilly, R. K. Anisotropic particles with patchy, multicompartment and Janus architectures: preparation and application. Chem. Soc. Rev. 40, 2402–2416 (2011).
Chen, J., Lai, Y., Wan, D., Jin, M. & Pu, H. Cooperative entrapment of xanthene dyes by a core-engineered unimolecular micelle. Macromol. Chem. Phys. 214, 1817–1828 (2013).
Arkas, M. & Tsiourvas, D. Organic/inorganic hybrid nanospheres based on hyperbranched poly(ethylene imine) encapsulated into silica for the sorption of toxic metal ions and polycyclic aromatic hydrocarbons from water. J. Hazard. Mater. 170, 35–42 (2009).
Wan, D. C., Ohta, S., Kakuchi, T. & Satoh, T. A hydrophilic unimolecular nanocapsule with cyclodextrin moieties in the core: chemically triggered on-demand release and pH-response. Soft Matter 7, 6422–6425 (2011).
Lan Chun, C., Lee, J.-J. & Park, J.-W. Solubilization of PAH mixtures by three different anionic surfactants. Environ. Pollut. 118, 307–313 (2002).
Dhas, N. A. & Suslick, K. S. Sonochemical preparation of hollow nanospheres and hollow nanocrystals. J. Am. Chem. Soc. 127, 2368–2369 (2005).
Acknowledgements
JD and DW thank the financial support from NSFC (21074094, 21174107, 21374080 and 51273149). JD also thanks the supports from eastern scholar professorship and Shanghai 1000 plan.
Author information
Authors and Affiliations
Contributions
F.C. characterized the adsorbent under the supervision of D.W. Q.G. conducted selectivity experiments and B.E.T. measurement. K.Z. did the fluorescence experiments. Q.L. and F.W. demonstrated the water purification by a column. H.L. drew the artwork. D.W. carried out other experiments with assistance from M.J. and H.P. H.W. proofed English. J.D. led the project, supervised Q.G., H.L., K.Z., Q.L. and F.W. analyzed the data and wrote the paper with assistance from D.W.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information for publication
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Wan, D., Chen, F., Geng, Q. et al. A multifunctional azobenzene-based polymeric adsorbent for effective water remediation. Sci Rep 4, 7296 (2014). https://doi.org/10.1038/srep07296
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07296
This article is cited by
-
Dyes removal from water using polymeric nanocomposites: a review
Environmental Chemistry Letters (2023)
-
Nanocomposite based on poly(lauryl acrylate)-grafted Fe3O4 for polycyclic aromatic hydrocarbon removal from water
Iranian Polymer Journal (2022)
-
Polymer nanodisks by collapse of nanocapsules
Science China Chemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.