Abstract
The ability of the rapid-capacitive discharge approach to access optimal viscosity ranges in metallic glasses for thermoplastic processing is explored. Using high-speed thermal imaging, the heating uniformity and stability against crystallization of Zr35Ti30Cu7.5Be27.5 metallic glass heated deeply into the supercooled region is investigated. The method enables homogeneous volumetric heating of bulk samples throughout the entire supercooled liquid region at high rates (~105 K/s) sufficient to bypass crystallization throughout. The crystallization onsets at temperatures in the vicinity of the “crystallization nose” were identified and a Time-Temperature-Transformation diagram is constructed, revealing a “critical heating rate” for the metallic glass of ~1000 K/s. Thermoplastic process windows in the optimal viscosity range of 100–104 Pa·s are identified, being confined between the glass relaxation and the eutectic crystallization transition. Within this process window, near-net forging of a fine precision metallic glass part is demonstrated.
Similar content being viewed by others
Introduction
Since their discovery, one of the most intriguing features of metallic glasses has been their ability to be formed “thermoplastically” above the glass transition temperature. This “thermoplastic forming” ability was widely perceived as finally bridging the gap between the manufacturing of metals and that of plastics and oxide glasses. Metallic glasses are kinetically frozen supercooled liquids and thus can be heated above their glass transition temperature where they can relax and recover the properties of the metastable liquid. Since glass-forming liquids have a well-defined equilibrium viscosity that depends strongly on temperature, it was thought to be possible to “dial in” to a viscosity simply by equilibrating the liquid at a certain temperature. As known from plastics processing, the optimum viscosity range for thermoplastic forming is typically 100–104 Pa·s1, which for metallic glasses implies a processing temperature in the supercooled liquid region well above the glass-transition temperature Tg (where the viscosity is on the order of 1012 Pa·s) and also substantially above the crystallization temperature Tx (where the metallic glass crystallizes when heated at a rate of ~1 K/s).
Thermoplastic forming of metallic glass was first proposed by Saotome et al., who reported micro-forming a metallic glass in the supercooled liquid region between Tg and Tx to produce micro-electro-mechanical components2,3,4,5,6,7. Following Saotome's approach, other authors exploited conventional thermoplastic methods used in the processing of plastics and conventional oxide glasses, such as blow molding8 and injection molding9, to process bulk articles. Theoretical approaches have also been conceived aiming to quantify the thermoplastic formability of metallic glasses between Tg and Tx10,11,12. These theoretical approaches are based on estimates of the flow capacity between Tg and Tx and involve integration of the viscosity function within that range. They essentially assume that flow would initiate at Tg and terminate at Tx, thereby rendering the difference ΔT = Tx − Tg a key parameter in quantifying formability. These models have inspired scientists to develop new bulk-glass forming alloys that demonstrate higher and higher ΔT in the hope of further improving metallic glass formability to levels comparable to plastics or conventional oxide glasses13. But even metallic glasses with very high ΔT values (as high as 165 K)14 have viscosities at Tx not lower than 106 Pa·s, i.e. still considerably higher than the optimum “thermoplastic” range. Unlike covalently and polymerically bonded glasses (e.g. silicates and plastics), metallic glasses are inherently unstable against crystallization; their centrosymmetric metallic bonding causes them to crystallize rather rapidly when the viscosity drops below 106 Pa·s. These limitations are well quantified by the “thermoplastic formability” models10,11,12.
To work around these limitations and enable access to the optimum thermoplastic viscosity range 100–104 Pa·s, the metallic glass must be heated extremely rapidly to temperatures high enough in the undercooled liquid region on a time scale much shorter than that associated with the onset of crystallization. Preferably, the heating rate utilized must be high enough to suppress crystallization throughout the undercooled liquid region. For typical bulk-glass formers, the “critical heating rate” to bypass crystallization by heating through the entire undercooled region (i.e. from Tg to the melting point) is known to be on the order of 104 K/s 15 and can be as low as ~102 K/s for very robust glass formers16. But conventional heating methods, where heat is introduced at the boundaries, are incapable of heating the entirety of bulk-glass samples at such high rates due to a sluggish thermal relaxation. Consider for example an amorphous rod with diameter d = 0.5 cm and thermal diffusivity α = 0.03 cm2/s (typical for metallic glasses) heated very rapidly over a surface skin (e.g. by high frequency induction). The relaxation time would be τ = (d/2)2/α ~ 1 s. Hence, to raise the centerline temperature by ~102 K the heating rate would be of order 102 K/s regardless of how rapidly the surface is heated. Such a heating rate is significantly lower than “critical heating rates” for typical glass formers and barely on the order of those for very robust bulk-glass formers. Therefore, 5-mm diameter rods of typical bulk-glass formers would crystallize at the core (and eventually throughout) irrespective of how rapidly their surface is being heated, while 5-mm diameter rods of very robust bulk-glass formers may remain marginally amorphous or barely crystallize.
Recently, a Joule heating approach has been introduced by Johnson et al17 that generates heat “volumetrically” through a metallic glass such that it overcomes the limitations imposed by thermal relaxation. In this approach, electrical current generated by discharging a capacitor across the sample passes uniformly through the sample enabling heating of the sample homogeneously throughout via dissipation of electrical energy. Since heating is volumetric, the transient temperature distribution is essentially homogenous and free of any gradients. Consequently, the sample is heated homogenously at a rate governed by the internal time constant of the electrical discharge circuit, i.e. by the current rise time. As such, the rate of homogeneous heating of a metallic glass can be controlled almost entirely by the parameters of the electrical circuit, i.e. by the capacitor discharge time constant. Capacitor discharge time constants can be varied enormously over many orders of magnitudes, covering ranges from microseconds to seconds. This allows a broad range of homogeneous heating rates that could be applied to entirely bypass crystallization (i.e. exceed critical heating rates) in a broad range of metallic glasses, from marginal glass formers to highly robust bulk-glass formers. Therefore, by properly designing the capacitor discharge characteristics, the entire undercooled liquid region of a metallic glass could be accessed for processing independent of the glass stability against crystallization, where any desired viscosity (e.g. in the range of 100–104 Pa·s) can be “dialed in” simply by selecting a discharge voltage. This is a powerful method that has the potential to entirely overcome the limitations imposed by the rapidly intervening crystallization during heating of metallic gasses.
In the work by Johnson et al.17 it was demonstrated that the rapid capacitive discharge heating method enables near-net thermoplastic shaping by injection molding. In the current work we use Zr35Ti30Cu7.5Be27.5 as a model bulk-glass former to explore the thermoplastic process window throughout the undercooled liquid region. Specifically, we employ high-speed infrared thermography and pyrometry to track the evolution of temperature distribution in ohmically heated bulk samples and accurately assess process windows defined between glass transition and crystallization by identifying the signatures for glass relaxation and liquid crystallization. By plotting the onsets of crystallization at the various temperatures in the undercooling region, the TTT diagram on heating is constructed. Lastly, thermoplastic forming by near-net forging in the optimum viscosity range of 100–104 Pa·s is presented.
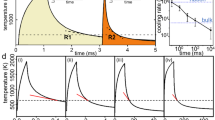
The temperature history and heating evolution in Zr35Ti30Cu7.5Be27.5 rods and strips undergoing ohmic heating at various discharge voltages were monitored by a high-speed pyrometer and a high-speed infrared imaging camera. The temperature history and heating evolution in the rod at a discharge voltage of 131.4 V associated with an energy density of 1883 J/cc is presented in Fig. 1. In Fig. 1A, the local temperature response reveals a heating rise time of about 2 ms, over which the heating process saturates as the current pulse fades and the temperature stabilizes at about 530°C. At this temperature the liquid viscosity is anticipated to be about 102 Pa·s13. The signature of a dynamic glass transition is revealed at approximately 475°C, which is 170°C higher than the calorimetric glass transition (measured at 20 K/min) for this glass13. This is a consequence of a very high heating rate, estimated to be of order 105 K/s. Following the cessation of heating at 530°C, the temperature appears to remain fairly stable for a period of about 3 s. Just after 3 s a fairly sharp recalescence event is evident in the pyrometer trace, as the sample begins to crystallize at the monitored location. In Fig. 1B, these events are revealed over a spatial range in the rod. During heating as well as after the heating process saturates, the temperature appears fairly uniform and stable across the entire domain monitored. Uniform and stable temperature persists for a period of up to 3 s, beyond which a “nucleus” of heat evolves at the core of the sample, which rapidly spikes to over 640°C while it propagates outwards to consume the entire sample at a speed on the order of ~1 cm/s. The sharpness of the recalescence event, which is consistent with that seen in scanning calorimetry13, suggests that the liquid crystallizes by coupled eutectic growth. From Figs. 1A and 1B therefore, a “thermoplastic process window” of about 2–3 s at 530°C is revealed where the liquid viscosity is expected to be about 102 Pa·s.
The temperature-time crystallization onsets in the ohmic heating experiments performed at various discharge voltages over multiple samples were determined using the slope intercept method and are plotted in Fig. 2. The onsets obtained for the same glass at lower temperatures and longer times using differential scanning calorimetry13 are also superimposed in the plot of Fig. 2. Figure 2 represents a TTT diagram associated with the heating a metallic glass spanning across the entire undercooled liquid region and is the first such diagram studied using thermally equilibrated bulk samples. Previous attempts either utilized uniform heating of micrometer-thick ribbons15, or involved surface heating of bulk samples16. A “critical heating rate” of ~1000 K/s can be estimated from the TTT diagram, associated with a “nose” temperature of 593°C and a “nose” time of 0.3 s. Circling back to the discussion on thermal relaxation, the critical heating rate of Zr35Ti30Cu7.5Be27.5 is considerably higher than the centerline heating rate in a surface-heated 5-cm rod which would be limited by thermal relaxation. This suggests that a 5-cm rod of Zr35Ti30Cu7.5Be27.5 glass heated rapidly at the surface would likely crystallize before or right after it accesses the optimum viscosity range for thermoplastic processing.
TTT diagram on heating of Zr35Ti30Cu7.5Be27.5 metallic glass.
High temperature data were obtained by rapid capacitive discharge heating (present work), while low temperature data were gathered using differential scanning calorimetry13.
To demonstrate the potential of the rapid capacitive discharge method for thermoplastic processing, a near-net forging operation is attempted within the thermoplastic process window identified near 530°C. Unlike injection molding, where the electrodes also act as plungers17, in forging the ohmic heating process is decoupled from the shaping process, with the forging dies operating independently from the electrodes. The process is presented schematically in Fig. 3, which illustrates forging a screw from a metallic glass rod. The rod is ohmically heated via the electrodes, while the forging dies subsequently (while the rod remains at the process temperature) apply a deformational force to shape the rod into a screw while simultaneously quench the sample to revitrify it.
Forging of a 5 mm amorphous Zr35Ti30Cu7.5Be27.5 rod (shown Fig. 4A) into a threaded screw is performed using process conditions similar to those that gave rise to the process window around 530°C. A digital image of the as-processed rod is shown in Fig. 4B. The screw component of the rod that has undergone forging is the part in the middle. The unforged part of the rod that has free cooled in air shows significant oxidation, however, the forged part visibly appears entirely free of oxidation. Using x-ray diffraction it was also verified that the forged part is entirely amorphous. The sectioned forged screw part is presented in Fig. 4C, while Fig. 4D shows the screw with the flashing removed. To examine the near-net forging precision, the threads were imaged with scanning electron microscopy. In Fig. 5A, the threads on a standard steel 10–32 screw is presented. The threads on the forging die are presented in Fig. 5B while the threads on the forged metallic glass screw are presented in Fig. 5C. As can be seen, the forged metallic glass screw has finely replicated the die and the threads closely match those of the standard steel screw.
In conclusion, the rapid capacitive discharge heating method is demonstrated here to be a powerful method capable of overcoming the shortcomings of metallic glasses in thermoplastic processing. Specifically, the method is capable of applying homogeneous heating rates that could entirely bypass crystallization in a broad range of metallic glasses, from marginal glass formers to highly robust bulk-glass formers, enabling access to the entire undercooled liquid region where any desired viscosity could be “dialed in” for thermoplastic processing. Lastly, the application of the rapid discharge heating process for near-net shape processing of metallic glasses presented can have a profound impact on the commercialization and overall technology scale-up of metallic glasses. The demonstrated ability to thermoplastically process a very high strength metal into high-precision near-net shapes is already quite an achievement, as was highlighted in prior literature1,2,3,4,5,6,7,8,9. But the ability to conduct such thermoplastic processing in open air over millisecond timescales using shaping pressures comparable to those used in the processing of plastics and at temperatures below typical tempering temperatures for conventional tooling, as demonstrated here, represents a significant technological leap forward.
Methods
To generate the heating curves, amorphous Zr35Ti30Cu7.5Be27.5 rods 5-mm in diameter 5-cm in length and strips 1-mm in thickness, 7-mm in width and 4-cm in length were prepared by arc-melting in a water-cooled copper hearth under inert atmosphere followed by suction casting in a copper mold. A high speed infrared imaging camera (FLIR Corp., SC2500) with a spectral band from 0.9 to 1.7 μm outfitted with a bandpass filter allowing wavelengths from 1.5 to 1.9 μm was employed at frame rates of 250–994 frames/s to record the evolution of temperature distribution. An IMPAC IGA740-LO high-speed infrared pyrometer with a spectral band from 1.58 to 2.2 μm and a response time of 6 microseconds was also used to record temperature vs. time over a circular focal spot of ~1 mm diameter near the rod center. Both camera and pyrometer were calibrated simultaneously by tracking the melting of Pd43Ni10Cu27P20 alloy with a solidus temperature of 531°C, which is within the temperature range considered in this work. Using this method, emissivities of 0.285 and 0.26 were found for the infrared camera and pyrometer, respectively. The emissivities are believed to be roughly representative of Zr35Ti30Cu7.5Be27.5 and that the temperature error is expected to be limited to within ±20°C. Ohmic heating of the amorphous Zr35Ti30Cu7.5Be27.5 samples was performed using a 0.264 F capacitor bank with a current rise time of about 1 ms was used at voltages ranging between 40–140 V.
For the forging operation, a split die was fabricated from stainless steel with half of a 1.8 cm long 10–32 thread on each side. A 5 mm amorphous Zr35Ti30Cu7.5Be27.5 rod prepared as described above was placed between the dies and held in place with copper leads. A 0.792 F capacitor bank was charged to 94 V depositing 2080 J/cc of electrical energy into the sample with a current rise time of about 10 ms. The initiation of the capacitive discharge is detected by a sensor circuit measuring current in a portion of the discharge circuit. The detection of discharge activates a pneumatic valve to apply a force of 3 kN to the softened metallic glass via a pneumatic piston. A delay of approximately 10 ms occurs between the activation of the valve and contact between the die and the sample, due to mechanical constraints of the piston. The process was performed in open air.
References
Han, D. C. Rheology and Processing of Polymeric Materials: Polymer processing. (Oxford University Press, Oxford, 2007).
Saotome, Y., Itoh, K., Zhang, T. & Inoue, A. Superplastic Nanoforming of Pd-Based Amorphous Alloy. Scripta Mater. 44, 1541–1545 (2001).
Saotome, Y., Hatori, T., Zhang, T. & Inoue, A. The Micro-Formability of Zr-Based Amorphous Alloys in their Supercooled Liquid State and their Application to Micro-Dies. J. Mater. Proc. Tech. 113, 64–69 (2001).
Saotome, Y., Miwa, S., Zhang, T. & Inoue, A. Superplastic Micro/Nano-Formability of La60Al20Ni10Co5Cu5 Amorphous Alloy in Supercooled Liquid State. Mater. Sci. Eng. A 304, 716–720 (2001).
Saotome, Y. et al. The Micro-Nanoformability of Pt-Based Metallic Glass and the Nanoforming of Three-Dimensional Structures. Intermetallics 10, 1241–1247 (2002).
Saotome, Y., Noguchi, Y., Zhang, T. & Inoue, A. Characteristic Behavior of Pt-Based Metallic Glass Under Rapid Heating and its Application to Microforming. Mater. Sci. Eng. A 375–377, 389–393 (2004).
Sharma, P., Kaushic, N., Kimura, H., Saotome, Y. & Inoue, A. Nanofabrication with Metallic Glass – An Exotic Material for Nano-Electromechanical Systems. Nanotechnology 18, 035302 (2007).
Schroers, J., Pham, Q., Peker, A., Paton, N. & Curtis, R. V. Blow Molding of Bulk Metallic Glass. Scripta Mater. 57, 341–344 (2007).
Wiest, A., Harmon, J. S., Demetriou, M. D., Conner, R. D. & Johnson, W. L. Injection Molding Metallic Glass. Scripta Mater. 60, 160–163 (2009).
Kato, H. et al. Fragility and Thermal Stability of Pt- and Pd-based Bulk Glass Forming Liquids and their Correlation with Deformability. Scripta Mater. 54, 2023–2027 (2006).
Schroers, J. On the Formability of Bulk Metallic Glass in its Supercooled Liquid State. Acta Mater. 56, 471–478 (2008).
Pitt, E. B., Kumar, G. & Schroers, J. Temperature Dependence of the Thermoplastic Formability in Bulk Metallic Glasses. J. Appl. Phys. 110, 043518 (2011).
Duan, G. et al. Bulk Metallic Glass with Benchmark Thermoplastic Processability. Adv. Mater. 19, 4272–2475 (2007).
Wiest, A. et al. Zr-Ti-Based, Be-Bearing Glasses Optimized for Thermal Stability and Thermoplastic Formability. Acta. Mater. 56, 2625–2630 (2008).
Pogatscher, S., Uggowitzer, P. J. & Loffler, J. F. In-Situ Probing of Metallic Glass Formation and Crystallization upon Heating and Cooling via Fast Differential Scanning Calorimetry. Appl. Phys. Lett. 104, 251908 (2014)
Schroers, J., Masuhr, A., Johnson, W. L. & Busch, R. Pronounced Asymmetry in the Crystallization Behavior during Constant Heating and Cooling of a Bulk Metallic Glass-Forming Liquid. Phys. Rev. B 60, 11855 (1999).
Johnson, W. L. et al. Beating Crystallization in Glass-Forming Metals by Millisecond Heating and Processing. Science 332, 828–833 (2011).
Acknowledgements
Authors acknowledge partial support by the II–VI Foundation and by Glassimetal Technology.
Author information
Authors and Affiliations
Contributions
G.K. and J.P.S. conducted the kinetic experiments and imaging analysis, G.K., T.H., K.S., T.T. and G.C. conducted the forging experiments, G.K., M.D.D. and W.L.J. wrote the main manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kaltenboeck, G., Harris, T., Sun, K. et al. Accessing thermoplastic processing windows in metallic glasses using rapid capacitive discharge. Sci Rep 4, 6441 (2014). https://doi.org/10.1038/srep06441
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06441
This article is cited by
-
Designing color in metallic glass
Scientific Reports (2019)
-
Inductive flash-annealing of bulk metallic glasses
Scientific Reports (2017)
-
Shaping metallic glasses by electromagnetic pulsing
Nature Communications (2016)
-
Quantifying the origin of metallic glass formation
Nature Communications (2016)
-
Thermoplastic Micro-Forming of Bulk Metallic Glasses: A Review
JOM (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.