Abstract
Extensive surveillance of influenza A viruses in different avian species is critical for understanding its transmission. Here, a breeding colony of Little Egrets and Black-crowned Night Herons was monitored both serologically and virologically in a city park of Jiangxi in 2009. A portion of herons had antibodies against H7 (52%), H5 (55%) and H9 (6%) subtype avian influenza virus (AIV) in egg yolk samples and 45% had antibodies against different AIV serotypes (H5, H7 or H9) simultaneously. Greater numbers of samples with anti-AIV H5N1 recombination-4 (Re-4, clade 7) antibodies were measured compared with those containing anti-H5N1 Re-1 (clade 0) and Re-5 (clade 2.3.4) antibodies. Eight strains of H5 and 9 strains of H9 were isolated from poultry of nearby markets. These results indicate wild birds are at risk from infection and co-infection with H7, H5 and H9 subtypes. Investigation of wild bird infection might provide an early warning sign of potential novel AIVs circulating in the nearby poultry industry and even in human society.
Similar content being viewed by others
Introduction
Avian influenza (AI) is a respiratory disease of domestic poultry and wild birds caused by influenza virus A of the family Orthomyxoviridae1. Influenza A viruses are classified into subtypes based on the antigenic properties of two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), expressed by virus particles. To date, 18 HA (H1–H18) and 11 NA (N1–N11) subtypes have been identified2, but infections with either H5 or H7 subtype virus are of most concern because of the potential for these viruses to mutate into a highly pathogenic form. Direct contact with infected poultry or contaminated objects is considered the main route of human infection3,4. However, some human infections are not associated with diseased poultry, as they have occurred in locations with no poultry AI outbreak5,6. Thus, extensive surveillance of influenza viruses in different avian species, in addition to domestic poultry, is critical for understanding the transmission of AI.
The role of migratory birds in AI outbreaks has been extensively discussed7. Herons (Aves: Ardeidae) are waterfowl that are widespread throughout China. Black-crowned Night Herons (Nycticorax nycticorax) are distributed among all provinces of China except for Xizang and both resident and summer migrant herons are present in Jiangxi provinces. Little Egrets (Egretta garzetta) are present in 22 provinces of China and only migrate in the summer to Jiangxi provinces8. Herons are susceptible to AI virus (AIV). In the Donana National Park, Spain, 3.1% of grey herons were infected with influenza A virus9. H5N1 virus was isolated from dead Little Egrets and Grey Herons in Penfold Park, Hong Kong in late 200210. In Cambodia, H5N1 killed 18% (2/11) of all Little Egrets between December 2003 and January 200411. Another report showed that striated herons were H5N1 seropositive in Java, Indonesia12. In China, from June 2004 to May 2005, Little Egrets and Black-crowned Night Herons were infected with AIV subtypes H2, H9 and H10 and maintained low-level antibodies against AIV13. Migratory ardeids can travel hundreds of miles and Little Egrets can hunt for prey up to 13 km from their own colonies14. Study of the AIV infection status of herons may provide important information on the role of herons in the spread of AIV.
Tianxiang Park is a city park in Nanchang, Jiangxi Province. The park is a natural wetland and an important breeding, overwintering and resting place for migratory birds. There are approximately 32 species of wild birds, totaling 180,000 birds. Among them, Little Egrets and Black-crowned Night Herons are the major breeding species. These birds have frequent contact with visitors to the park, thus posing a potential risk for transmitting pathogens to humans.
Determination of AIV infection status of a bird flock is determined through serum antibody testing. Serum collection is routine for broilers, while for layers, eggs are a rich source of antibodies and have therefore been used for serologic monitoring of egg layers against various poultry diseases. During the 1983–1984 H5N2 AIV outbreaks in the northeastern United States, egg yolk extraction procedures were developed and used to determine the influenza status of egg layers15. In 2003, egg yolk was used as an alternative sample source to serum for the detection of anti-influenza antibodies in white leghorn hens in a surveillance program16. In 2010, egg yolk was validated as an alternative source to serum for antibody monitoring of laying breeder ducks17. Thus, diagnosis and disease monitoring can be accomplished by evaluating egg yolk samples as an alternative to serum, with the advantage of being easier to sample and less intrusive for adult birds.
This study investigated the AIV infection status of Little Egrets and Black-crowned Night Herons in Tianxiang Park, Jiangxi province of China (Figure 1), by detecting anti-AIV antibodies in egg yolk and isolating AIV from feces. The objective of this study was to improve our understanding of the role of herons in the spread of AIV and provide information for the development of efficient prevention and control measures to reduce the transmission of AIV from wild birds to poultry and humans. The study indicates that the potential infection and co-infection of wild birds with multiple subtypes of AIV might spread virus to poultry in other locations.
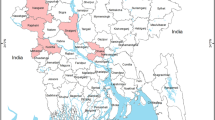
Study area in the Poyang Lake wetland of Jiangxi province showing (a) map of China, (b) migratory path of herons along Poyang Lake wetland and Hong Kong and (c) false color display of imagery presenting Tianxiang Park and four nearby live bird markets.
The maps and the imagery are generated by the software ArcGIS10 (Esri, Redlands, CA, USA). Forests and green vegetation are shown in red, lakes and rivers are shown in black, while urban area is shown in greyish tone and transportation and roads are shown in lighter gray tone. Tianxiang Park is located in an urban agglomerate, which is densely populated.
Results
Serological analyses of AIV infection in herons
A total of 64 egg yolk samples were examined for anti-AIV antibodies by HI test. HI titers of egg yolk samples are summarized in Table 1. HI titers of antibodies against H5 and H7 subtypes AIV in Little Egrets or Black-crowned Night Herons were mostly between 4 and 6, while HI titers against H9 subtype AIV were mostly less than 4.
Based on the seropositive standard (HI antibody titer to AIV ≥ 4) advocated by the World Health Organization (WHO) and Office International des Epizooties (OIE), 3.45% (1/29) of Little Egrets and 14.29% (5/35) of Black-crowned Night Herons egg yolk samples were positive for H5 subtype AIV, 6.90% (2/29) and 5.71% (2/35), respectively were positive against H7 subtype AIV and none (0/29, 0/35) were positive against H9 subtype AIV (Table 2).
Existence of multiple serotype antibodies in single individual herons
Among the egg yolk samples collected and tested, we found that some herons had antibodies against different serotypes of influenza viruses simultaneously (Table 2). The rate of seropositivity against both H5 and H7 AIV subtypes in Little Egrets and Black-crowned Night Herons was 44.83% (13/29) and 34.29% (12/35), respectively and against triple H5, H7 and H9 AIV subtypes was 13.79% (4/29) and 0% (0/35) (Table 2).
Detection of antibodies against relevant clades of AIVs (H5N1)
If herons were infected with the AIV H5 subtype, we wanted to determine whether it was similar to the prevalent strain. In the past few years, the AIV H5 subtype belonging to clade 7, clade 2.3.4 and clade 2.3.2 was dominant in poultry in China. Although A/goose/Guangdong/1/96 belonging to clade 0 has disappeared, it was the precursor of other virus strains18. We tested the egg yolk antibodies against three clade (clade 0, clade 7 and clade 2.3.4) viruses by HI test. Three vaccine strains, H5N1 recombination-1 (H5N1 Re-1), H5N1 Re-4 and H5N1 Re-5 were used as antigens to test the egg yolk samples. In Little Egrets or Black-crowned Night Herons, most samples had anti-AIV H5N1 Re-4 antibodies (Table 3). Only 5 Little Egret and 3 Black-crowned Night Heron samples contained both anti-AIV H5N1 Re-1 and H5N1 Re-4 antibodies and none and 1 sample had anti-AIV H5N1 Re-5 antibodies in Little Egrets or Black-crowned Night Herons, respectively.
Virus isolation from herons and nearby poultry
In 2009, we collected cloacal and oropharyngeal swabs from 93 chickens and 123 ducks (432 total swabs) in September and from 61 chickens and 80 ducks (282 total swabs) in December, from four live poultry markets near Tianxiang Park. Virus isolation from all swab samples was performed. Table 4 shows that 7 H5 subtype AIV and 6 H9 subtype AIV were isolated in September 2009 and 1 H5 subtype AIV and 3 H9 subtype AIV were isolated in December 2009. However, no H7 subtype AIV was isolated from any sample.
Virus isolation from all 58 feces samples were tested for AIV by inoculation in embryonated SPF eggs. Despite continued passaging, hemagglutinin-positive agents were not detected and the amplification of AIV gene by RT-PCR was also negative.
Discussion and Conclusions
There is no doubt that wild migratory birds can be infected and may spread disease to local poultry flocks19. Because of the ecological importance of AIV to animal and human health, we investigated AIV infections in Little Egrets and Black-crowned Night Herons in Tianxiang Park. The antibody positive frequency for H5 and H7 subtypes was high in both Little Egrets and Black-crowned Night Herons. As no vaccination program for avian influenza was performed in these wild birds, the surveillance results reflect the natural situation of AIV in heron populations. Although most H5 and H7 viruses induce low pathogenic avian influenza (LPAI), most highly pathogenic avian influenza (HPAI) viruses have been restricted to subtypes H5 and H7. AI is an economically important disease for the poultry industry because of losses of flocks and HPAI can cause up to 100% mortality. Furthermore, HPAI outbreaks not only lead to enormous economic impacts but also threaten human health. Since November 2003, 667 human infections with HPAI H5N1 virus in 15 countries have occurred and there have been 393 deaths reported as of June 201420. Since March 2013, there has been an emerging novel influenza A (H7N9) infection with 450 confirmed human cases and as of June 2014 there have been 165 deaths in China21. Because the risk is highly dependent on the prevalence of AIV infection in natural host-reservoir systems, it is critically important to understand the biology of virus prevalence in the wild22,23.
Furthermore, HI test results showed that the positive rate of samples infected with both H5 and H7 subtypes was high, 44.83 and 34.29% in Little Egrets and Black-crowned Night Herons, respectively. Because of the serological methods used, the detection of multiple antibodies in a given individual does not necessarily suggest co-infection. When multiple antibodies are detected, it is not clear whether the viruses infected the host simultaneously or sequentially. If birds are infected with the two virus subtypes simultaneously, recombination may occur and produce new pathogenic AIV, causing a pandemic in the poultry industry and issues for human health. High-throughput next-generation sequencing of isolated virus from poultry in southern China also showed that some poultry were co-infected with different virus genotypes23, thus further supporting the idea of co-infection of herons with different virus subtypes. HI test results from a sentinel location in the Poyang Lake area, an aquatic bird-breeding farm, showed the positive rate of samples infected with H7 subtypes was as high as 58.06%. Wild birds may serve as sentinel hosts. Virological and serological investigation of AI infection in wild birds may provide important information for the surveillance of novel, emerging AIVs.
The seropositive results indicated that herons might be infected with AIV a few weeks or months before breeding. These herons migrate from Hong Kong to Tianxiang Park to breed each year and return as the weather becomes colder. The herons we investigated might become infected with AIV in Hong Kong or Nanchang or on the migration route and potential routes of infection include contact with wild birds, human mobility and exposure to nearby live poultry markets. Prior to this investigation there have been no reports regarding avian influenza outbreaks in the Jiangxi province or Hong Kong. From GenBank we observed that H5N1 viruses were isolated from grey herons and Little Egrets in Hong Kong in 2007 and from Black-crowned Night Herons, Great Egrets and Grey Herons in Hong Kong in 2008. However, only one strain, H9N2 virus, was isolated from humans in Hong Kong in 2008 and there have been no H7 subtype AIVs isolated in Hong Kong in recent years. In Southern China, H5N1 AIV was isolated from a condor in Guangdong in 200324; H5N2 and H5N1 AIV were isolated from parrots in Guangdong in 2004 and 200525,26, respectively; several H9N2 AIVs were isolated from water and fowl feces in the Dongting Lake wetland in 200727 and from broilers in Fujian in 200928; 4 H7N3AIVs were isolated from domestic ducks in Zhejiang in 201129; H6N2 AIV was isolated from a chicken in Guangdong in 201130; recombinant H9N2 AIV from fresh egret feces in the wetland in 201231; and H10N8 AIVs were isolated from water samples of the wetland in 201132 and from a duck in Guangdong in 201233. Prior reports showed that from January 2000 through April 2001, multiple H3N6, H9N2, H2N9, H3N3 and H4N6 AIVs and single H1N1 and H3N2 AIVs were isolated from a Nanchang live poultry market34. Twenty-four influenza A viruses of five HA subtypes (H1, H3, H4, H6 and H10) were isolated from apparently healthy migratory ducks and geese at Poyang Lake wetland during November 2003 to March 2005. Six HPAI H5N1 viruses were isolated from apparently healthy migratory ducks at Poyang Lake wetland on two sampling occasions (January and March 2005) just before they migrated northward35. Furthermore, H5N3 was isolated from ducks in 2003, H7N7 from ducks in 200336, H5N1 from migratory ducks in 2005 and H5 virus from chickens in 2008 in Jiangxi Province37. These results strongly support the idea that H5, H9 and some other AIV subtypes recently existed in Hong Kong and southern China, although only one H7N7 was isolated from ducks in Jiangxi in 2003 and 4 H7N3AIVs isolated from domestic ducks in Zhejiang in 2011. Therefore, we speculate that the herons we investigated might become infected with AIV in Hong Kong or Nanchang or on the migration route, but could not conclusively identify the source of infection.
Additionally, these herons could spread AIV during their migration. Therefore, after this investigation we collected oropharyngeal and cloacal swabs from poultry in four live poultry markets near Tianxiang Park. H5 and H9 subtype AIVs were isolated from these samples. Although the antibody positive frequency for the H7 subtype was higher in both Little Egrets and Black-crowned Night Herons, these birds did not spread H7 AIV to local poultry, but possibly to the aquatic birds in a neighboring breeding farm.
Since the 2004 vaccination program, H5N1 Re-1 has been extensively used in China, until A/chicken/Shanxi/2/2006 (CK/SX/06) and A/chicken/Ningxia/24/2006(CK/NX/06) were isolated in 2006. The HI titers differed 64-fold between A/Goose/Guangdong/1/96 and these two viruses. Therefore, vaccine H5N1 Re-4 was used in those affected provinces, mainly in northern China. Since 2005, genetic analysis revealed an H5N1 influenza variant, Clade 2.3.4 (Fujian-like sub-lineage) had emerged and become predominant, replacing those previously established multiple sub-lineages in different regions of southern China38, yielding the H5N1 Re-5 vaccine. Our results showed that in either Little Egrets or Black-crowned Night Herons most of the samples contained anti-AIV H5N1 Re-4 antibodies, some samples had both anti-AIV H5N1 Re-1 and H5N1 Re-4 antibodies, but few had anti-AIV H5N1 Re-5 antibodies. Thus, this flock of birds that live freely in the wild might have close contact with virus infected poultry or wild birds, or contaminated environments in their living places or along their migratory route. To reduce avian influenza outbreak in poultry farms, AIV H5 Re-4 or AIV H5 Re-1 and Re-4 bi-linked vaccines should be recommended for use along the birds' migratory route in China.
Most wild birds did not develop noticeable clinical symptoms after AIV infection, but could retain persistent virus39. Therefore, they represent a virus reservoir that can infect poultry. Previous reports indicated that outbreaks of AIV in poultry have spatial and temporal relationship with wild birds40,41,42,43 and as such, virus-carrying migratory birds can spread AIV around the world. Live poultry market then acts as a relay station for AIV circulation in the case of H7N9 and H10N8, as it provides environments conducive to genomic segment re-assortment, gene mutation and interspecies transmission of AIVs among wild and domesticated animals44,45. Migratory birds might have served as an important origin of the chain infection in China. Thus, investigation of the AIV infection status of wild birds has importance to viral transmission mode.
Methods
Ethics statement
Collection of samples from herons in the Tianxiang park was approved by the Department of Wildlife Conservation of the State Forestry Administration (Permit number: GYXZ-2009-12) concerned with protection of wildlife. The owner of the land gave permission to conduct the study on this site. Swabs collected from the poultry at live bird markets were approved by the owners of live bird markets. The protocol of the study was conducted according to the guidelines of animal welfare of the World Organization for Animal Health and approved by the institutional ethics committee in the School of Environment, Tsinghua University, the Animal Care and Use Committee at the Beijing Normal University, Wuhan Institute of Virology and China Agricultural University (Permit number: 20090318).
Collection of eggs and feces
Tianxiang Park (N 28.66, E 115.95) is a nature park with high population density of wild birds, situated about 4 km from Nanchang City and covers an area of 105 km2 containing wetland, pool and marsh areas (Figure 1). There are four live bird markets, three lakes and many villages within 13 km of Tianxiang Park. Trees are used as nighttime roosts by Little Egrets and Black-crowned Night Herons and more than 200 birds may rest in one tree. Chinese Elm (Ulmus parvifolia Jacq.), Paper Mulberry (Broussonetia papyrifera Linn. Vent.), Babylon Willow (Salix babylonica Lin.), Chinaberry (Melia azedarach L.), Camphor Laurel (Cinnamomum camphora L.), White Mulberry (Morus alba Linn.) and Canada Poplar (Populus canadensis Moench) are the main species of trees in Tianxiang Park. Little Egrets and Black-crowned Night Herons are common species found in Tianxiang Park as they migrate there each year for breeding, before returning to Hong Kong as the weather becomes colder. Some of the birds were not migratory, but were resident all year round. A total of 64 eggs were collected in the breeding season between 16th April and 10th May 2009. Among the 64 samples, 29 were collected from Little Egrets (45.3%) and 35 were collected from Black-crowned Night Herons (54.7%). All eggs collected were labeled, weighed and measured. Additionally, 58 feces samples were collected under the nests where the eggs were obtained.
Preparation of egg yolk
Eggs were individually cracked and the egg white was separated from the yolk. The yolk sac was ruptured with a needle and the contents were collected with a syringe under sterile conditions. Then the yolk was mixed with an equal amount of 0.01 M phosphate buffered saline (PBS; pH 7.4) and homogenized. The mixture was left for 1 h at room temperature followed by centrifugation at 1,000 × g for 30 min. The supernatant was collected and stored at −80°C until use.
Hemagglutination inhibition (HI) test
Antibodies against AIV in egg yolks were measured by HI test using standard procedures46. Briefly, 25 μl of egg yolk samples were serially diluted using a 2-fold dilution series in V-shaped microtiter plates. Then the samples were mixed with 4 HA units of antigen and incubated at room temperature for 30 min. Then, 25 μl of 1% SPF chicken erythrocytes were added to each well and incubated at room temperature for 30 min. Each test included a negative control without antigen. HI titers were expressed as log2 of the reciprocal of the highest dilution of samples causing complete inhibition of 4 HA units of antigen. Titers ≥ 4 were regarded as positive. Three vaccine strains, H5N1 Re-1, H5N1 Re-4 and H5N1 Re-5 were generated through reverse genetics by Harbin Veterinary Research Institute, China47,48. AIV H5N1 Re-1 was yielded using six genes from the influenza A virus A/Puerto Rico/8/1934(H1N1) (PR8) and the HA and NA genes of A/Goose/Guangdong/1/96, which belong to clade 0. The HA and NA genes were mutated and multiple basic amino acids were deleted at the cleavage sites. Similarly, H5N1 Re-4 used six genes of PR8, the HA gene of A/CK/Shanxi/2/2006 that belongs to clade 7 and the NA gene of A/Goose/Guangdong/1/96. H5N1 Re-5 used six genes of PR8, the HA gene of A/Duck/Anhui/1/2006 belonging to clade 2.3.4 and the NA gene of A/Goose/Guangdong/1/96. In this study, we limited our investigation to AI subtypes that may be pathogenic to poultry and humans. Standard AIV positive sera and antigens of H5, H7 and H9 subtypes and vaccine strain H5N1 Re-1, H5N1 Re-4 and H5N1 Re-5 were supplied by the Harbin Veterinary Research Institute, China.
Collection of swabs from poultry in live bird markets
To investigate the potential impact of herons on nearby poultry, we collected cloacal and oropharyngeal swabs from apparently healthy poultry in four live bird markets near Tianxiang Park. A total of 216 and 141 pairs of swabs were collected in September and December 2009, respectively. The samples were eluted with 2.0 ml DMEM containing 0.2% fetal bovine serum, penicillin (2,000 units/ml) and streptomycin (2,000 units/ml). The samples were kept at 4°C until they were transported to the laboratory and were then stored at −80°C until use.
Virus isolation
The samples were thawed, shaken for 10 min and centrifuged at 6,000 × g/min for 10 min at 4°C, then the supernatant was mixed with PBS containing antibiotics (penicillin, 10,000 units/ml; streptomycin, 10,000 units/ml; gentamycin, 2,000 μg/ml and nystatin, 500 units/ml) for 24 h at 4°C. Inoculated material (0.2 ml) was inoculated into the allantoic cavities of 9 to 11-day-old specific pathogen free (SPF) embryonated chicken eggs. After incubation at 37°C for 72 h or immediately after embryos died, allantoic fluid from inoculated eggs was collected and tested for HA activity. Either hemagglutinin-positive or -negative allantoic fluids were passaged for three generations on embryonated eggs. To remove bacterial contamination, allantoic fluids were passed through 0.2-μm-pore-size syringe filters. After the third passage, the harvested allantoic fluids were detected for AIV by HA test.
RT-PCR
For hemagglutinin-negative samples, we performed genetic analysis. Viral RNA was extracted from the positive allantioc fluids using the RNeasy Mini Kit (QIAGEN, Hilden, Germany). Reverse transcription reactions were performed by SuperScript®III First-Strand Synthesis System For RT-PCR Kit (Invitrogen, Carlsbad, CA, USA) using Uni-12 primer49.
References
Alexander, D. The epidemiology and control of avian influenza and Newcastle disease. J. Comp. Pathol. 112, 105–126 (1995).
Wu, Y., Wu, Y., Tefsen, B., Shi, Y. & Gao, G. F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends. Microbiol. 22, 183–191 (2014).
Guan, Y. et al. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. USA. 101, 8156–8161 (2004).
Brankston, G., Gitterman, L., Hirji, Z., Lemieux, C. & Gardam, M. Transmission of influenza A in human beings. Lancet. Infect. Dis. 7, 257–265 (2007).
Yu, H. et al. Human influenza A (H5N1) cases, urban areas of People's Republic of China, 2005–2006. Emerg. Infect. Dis. 13, 1061 (2007).
Wang, H. et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 371, 1427–1434 (2008).
Alexander, D. J. A review of avian influenza in different bird species. Vet. Microbiol. 74, 3–13 (2000).
Zhu, X. & Zou, X. P. The herons of China. (China Forestry Publishing House 2001).
Astorga, R. et al. Avian influenza in wild waterfowl and shorebirds in the Doñana National Park: Serological survey using the enzyme-linked immunosorbent assay. Avian. Pathol. 23, 339–344 (1994).
Ellis, T. M. et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian. Pathol. 33, 492–505 (2004).
Desvaux, S. et al. Highly pathogenic avian influenza virus (H5N1) outbreak in captive wild birds and cats, Cambodia. Emerg. Infect. Dis. 15, 475 (2009).
Stoops, A. C. et al. H5N1 surveillance in migratory birds in Java, Indonesia. Vector. Borne. Zoonotic. Dis. 9, 695–702 (2009).
Chen, H. X. et al. Seroprevalance and identification of influenza A virus infection from migratory wild waterfowl in China (2004–2005). J. Vet. Med. B. Infect. Dis. Vet. Public. Health. 53, 166–170 (2006).
Berny, P. et al. Impact of local agricultural and industrial practices on organic contamination of little egret (Egretta garzetta) eggs in the Rhone delta, southern France. Environ. Toxicol. Chem. 21, 520–526 (2002).
Pearson, J. et al. Characterization of Newcastle disease virus (avian paramyxovirus-1) isolated from pigeons. Avian. Dis. 31, 105–111 (1987).
Beck, J., Swayne, D., Davison, S., Casavant, S. & Gutierrez, C. Validation of egg yolk antibody testing as a method to determine influenza status in white leghorn hens. Avian. Dis. 47, 1196–1199 (2003).
Jeong, O.-M. et al. Validation of egg yolk antibody based C-ELISA for avian influenza surveillance in breeder duck. Vet. Microbiol. 144, 287–292 (2010).
Abdel-Ghafar, A.-N. et al. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358, 261–273 (2008).
Webster, R. G., Peiris, M., Chen, H. & Guan, Y. H5N1 outbreaks and enzootic influenza. Emerg. Infect. Dis. 12, 3–8 (2006).
WHO. Influenza at the human-animal interface <http://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en/> Date of access: 27/06/2014 (2014a).
WHO. WHO RISK ASSESSMENT. Human infections with avian influenza A(H7N9) virus <http://www.who.int/influenza/human_animal_interface/influenza_h7n9/en/> Date of access: 27/06/2014 (2014b).
Capua, I. & Alexander, D. J. Avian influenza: recent developments. Avian. Pathol. 33, 393–404 (2004).
Yu, X. et al. Influenza H7N9 and H9N2 viruses: Coexistence in Poultry Linked to Human H7N9 Infection and Genome Characteristics. J. Virol. 88, 3423–343 (2014).
Jiao, P. et al. Full Genome Sequence of a Recombinant H5N1 Influenza Virus from a Condor in Southern China. J. Virol. 86, 7722–7723 (2012).
Jiao, P. et al. Complete genome sequence of an H5N2 avian influenza virus isolated from a parrot in Southern China. J. Virol. 86, 8890–8891 (2012).
Jiao, P. et al. Complete Genomic Sequence of an H5N1 Influenza Virus from a Parrot in Southern China. J. Virol. 86, 8894–8895 (2012).
Zhang, H., Xu, B., Chen, Q. & Chen, Z. Characterization of H9N2 influenza viruses isolated from Dongting Lake wetland in 2007. Arch. Virol. 156, 95–105 (2011).
Xie, Q. et al. Complete genome sequence of a novel H9N2 subtype influenza virus FJG9 strain in china reveals a natural reassortant event. J. Virol. 86, 10240–10241 (2012).
Jiao, P. et al. Complete Genome Sequence of an H7N3 Avian Influenza Virus Isolated from Ducks in Southern China. J. Virol. 86, 7724–7725 (2012).
Jiao, P. et al. Complete Genomic Sequence of a Novel Natural Recombinant H6N2 Influenza Virus from Chickens in Guangdong, Southern China. J. Virol. 86, 7717–7718 (2012).
Wang, B., Chen, Q. & Chen, Z. Complete Genome Sequence of an H9N2 Avian Influenza Virus Isolated from Egret in Lake Dongting Wetland. J. Virol. 86, 11939–11939 (2012).
Zhang, H., Xu, B., Chen, Q., Chen, J. & Chen, Z. Characterization of an H10N8 influenza virus isolated from Dongting lake wetland. Virol. J. 8, 42 (2011).
Jiao, P. et al. Complete Genome Sequence of an H10N8 Avian Influenza Virus Isolated from a Live Bird Market in Southern China. J. Virol. 86, 7716–7716 (2012).
Liu, M. et al. The influenza virus gene pool in a poultry market in south central China. Virology. 305, 267–275 (2003).
Chen, H. et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc. Natl. Acad. Sci. USA. 103, 2845–2850 (2006).
Campitelli, L. et al. Molecular analysis of avian H7 influenza viruses circulating in Eurasia in 1999–2005: detection of multiple reassortant virus genotypes. J. Gen. Virol. 89, 48–59 (2008).
Jiang, W.-M. et al. Molecular epidemiological surveys of H5 subtype highly pathogenic avian influenza viruses in poultry in China during 2007–2009. J. Gen. Virol. 91, 2491–2496 (2010).
Smith, G. et al. Emergence and predominance of an H5N1 influenza variant in China. Proc. Natl. Acad. Sci. USA. 103, 16936–16941 (2006).
Webster, R. G. & Govorkova, E. A. H5N1 influenza—continuing evolution and spread. N. Engl. J. Med. 355, 2174–2177 (2006).
Halvorson, D. et al. Epizootiology of avian influenza: simultaneous monitoring of sentinel ducks and turkeys in Minnesota. Avian. Dis. 27, 77–85 (1983).
Karunakaran, D., Hinshaw, V., Poss, P., Newman, J. & Halvorson, D. Influenza A outbreaks in Minnesota turkeys due to subtype H10N7 and possible transmission by waterfowl. Avian. Dis. 27, 357–366 (1983).
Wang, Y., Jiang, Z., Jin, Z., Tan, H. & Xu, B. Risk Factors for Infectious Diseases in Backyard Poultry Farms in the Poyang Lake Area, China. PLoS. One. e67366 (2013).
Yao, Y. et al. Characterization of low-pathogenic H6N6 avian influenza viruses in central China. Arch. Virol. 158, 367–377 (2013).
Gao, R. et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368, 10.1056/NEJMoa1304459 (2013).
Zhang, T. et al. Human Infection with Influenza Virus A (H10N8) from Live Poultry Markets, China, 2014. Emerg. Infect. Dis. http://dx.doi.org/10.3201/eid2012.140911 (2014).
Palmer, D. F., Dowdle, W. R., Coleman, M. T. & Schild, G. C. Advanced laboratory technicals for immunological diagnostic. Procedural guide Part 2: Haemagglutination-inhibition test. Immunology Series. 6, US Department of Health, Education and Welfare, P.H.S, Atlanta, 25–62 (1975).
Tian, G.-b. et al. The biological characteristics and immunogenicity of a H5N1 inactivated vaccine seed virus strain Re-4 generated from the avian influenza virus variant strain. Chin. J. Prev. Vet. Med. 9, 014 (2009).
Chen, H. H5N1 avian influenza in China. Sci. China. C. Life. Sci. 52, 419–427 (2009).
Hoffmann, E., Stech, J., Guan, Y., Webster, R. & Perez, D. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146, 2275–2289 (2001).
Acknowledgements
This research was supported by the Ministry of Science and Technology, China, National Research Program (2010CB530300, 2012CB955501, 2012AA12A407) and the National Natural Science Foundation of China (41271099).
Author information
Authors and Affiliations
Contributions
B.X., L.L. and G.W. conceived and designed the experiments. G.W., T.Z., X.L., Z.J., Q.J. and X.T. performed the experiments. G.W., T.Z., X.L., Z.J., Q.J., Q.C., X.T., Z.C., J.C., L.L. and B.X. analyzed the data. Q.C., X.T., Z.C., J.C., L.L. and B.X. contributed reagents/materials/analysis tools. G.W., T.Z., X.L., Z.J., Q.J., Q.C., X.T., Z.C., J.C., L.L. and B.X. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Wang, G., Zhang, T., Li, X. et al. Serological evidence of H7, H5 and H9 avian influenza virus co-infection among herons in a city park in Jiangxi, China. Sci Rep 4, 6345 (2014). https://doi.org/10.1038/srep06345
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06345
This article is cited by
-
Co-inections of domestic and wild birds with avian influenza and Newcastle disease viruses: implications for control and genetic mutations
Veterinary Research Communications (2020)
-
Risk analysis of H5N1 highly pathogenic avian influenza in poultry at the Poyang Lake area, China
Environmental Earth Sciences (2016)
-
Risk Distribution of Human Infections with Avian Influenza H7N9 and H5N1 virus in China
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.