Abstract
Space flight strongly moderates human immunity but is in general well tolerated. Elucidation of the mechanisms by which zero gravity interacts with human immunity may provide clues for developing rational avenues to deal with exaggerated immune responses, e.g. as in autoimmune disease. Using two sounding rockets and one manned Soyuz launch, the influence of space flight on immunological signal transduction provoked by lipopolysaccharide (LPS) stimulation was investigated in freshly isolated peripheral blood monocytes and was compared to samples obtained from on-board centrifuge-loaded 1 g controls. The effect of microgravity on immunological signal transduction is highly specific, since LPS dependent Jun-N-terminal kinase activation is impaired in the 0 g condition, while the corresponding LPS dependent activation of p38 MAP kinase remains unaffected. Thus our results identify Jun-N-terminal kinase as a relevant target in immunity for microgravity and support using Jun-N-terminal kinase specific inhibitors for combating autoimmune disease.
Similar content being viewed by others
Introduction
The constant threat of pathogenic organisms and the resulting evolutionary pressure to develop defences against these challenges, has resulted in an array of collaborative defensive cell systems. These consist, amongst others, of phagocytes and adaptive B and T cell systems which are collectively grouped under the broad denominator immunity. The human immune system, like all cell biological systems in our body, has developed in the continuous presence of the earth's gravitational field. Thus it is conceivable that acute absence of this external force, as occurs during space flight, would influence functionality in this system. Indeed since the early days of space exploration it has been evident that extra-atmospheric primates and humans experience reduced immune function1,2,3,4. Certainly, some of the observed effects on immune function can be related to environmental factors such as cosmic radiation5 and magnetic fields6. Furthermore, psychological factors such as stress and anxiety7 from living and working in a confined space, under cramped conditions and with inadequate lifestyle, nutritional regimen and disturbance of circadian rhythm8,9 may affect the immune system. Nevertheless, there are good reasons to believe that the weightlessness itself negatively influences immunocompetence of the human body10,11,12,13. Experimentation with crew members of space flights showed reduced anti-viral immunity15 and a landmark experiment conducted in Spacelab 1 in 1983 demonstrated a near total absence of activation of peripheral blood leukocytes upon exposure to concanavalin A (Con-A) when compared to identical ground controls4. The mechanistic basis of this effect however, remains largely obscure. A recent study employing DNA array analysis of Con-A and anti-CD28-stimulated T cells, on board of the international space station, showed that microgravity-exposed T cells were deficient in transcription of immediate early genes associated with the tumour necrosis factor alpha pathway. However, T cells stimulated in an on-board centrifuge, artificially generating a 1 g condition, did not show this effect. And thus the effects of spaceflight on the immune system are related to microgravity and are indeed not just a mere artefact resulting from stress4,14. The molecular pathways mediating these effects remain to be elucidated, but their knowledge could provide important clues on how to device better therapies for conditions associated with exaggerated activation of the immune system on earth, e.g. autoimmune disease or leukaemia.
Results
Experimental design
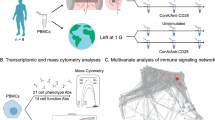
To identify the interaction of the immune system with spaceflight and microgravity in particular, we decided to investigate the effect of stimulation of primary isolated human peripheral blood monocytes with LPS in 0 g versus 1 g conditions during space flight. LPS is a relatively invariant surface structure of gram negative bacteria. Its recognition by TLR-4 forms an important part of the innate immune response16,17 and provokes highly reproducible responses and signal transduction in peripheral blood monocytes, which have been characterized in great detail18. To this end, after appropriate consent, blood was obtained from young healthy volunteers (aged 22–31) and monocytes were isolated using Ficoll density gradient centrifugation followed by classical adherence19. Monocytes were loaded in fully-automated culture units capable of administrating 100 ng/ml LPS or vehicle control followed by fixative at predetermined timelines. The loaded units were then placed in a specially designed module which maintained the ambient temperature at 37°C. Within the module, units were either placed on a static rack, permissive of exposing cells to changes in gravity or on a centrifuge that maintained cells at 1 g throughout the experiments. For our study we chose to initiate experimentation 1 min after the onset of microgravity by application of LPS. Cells were fixed with 4% formaldehyde after 5 min of LPS stimulation, as we have shown previously that this time point correlates with the most profound LPS-induced changes in monocyte kinase activity18. Figure 1 schematically shows the setup and timeline of the experiments involved. In this fashion, the experiment was executed three times. One time on a manned Soyuz flight launched from Baikanur in Kazakhstan and two times on a Maser sounding rocket launched from Esrange in Sweden. Stimulations were performed within 10 hour after blood was drawn. After recovery of the module, the material was investigated for space flight and microgravity-induced effects on LPS-induced signal transduction.
Summary of the experimental procedure and the timeline.
The experiment was repeated three times. The first experiment was performed as part of the 2004 Soyuz TMA-4 campaign. Under the name Kappa it was executed during a manned flight to the International Space Station. The experiment was repeated in 2005 during the Maser 10 mission (Amuse) and again in 2012 during the Maser 12 mission (Micimmun). Both missions used sounding rockets to carry the experiment into space. During a parabolic flight the rocket reaches an altitude of approximately 260 km allowing for 5 to 6 minutes of microgravity. The experiments are performed in automated climate chambers termed Flush Units. The Flush Units are placed within the BIM module. The module regulates ambient temperature aboard the rocket and provides electricity to the Flush Units during flight. As an onboard control the Flush Units can be placed in a centrifuge which spins at a speed equal to the force of gravity. The other units are placed on a static rack and are allowed to experience microgravity. Prior to the flight the monocytes are placed inside a rectangular chamber containing two glass slides to which the monocytes attach. At the start of microgravity a timed mechanism releases a spring plunger which replaces the medium in the culture chamber with medium containing LPS. At the end of microgravity a similar mechanism replaces the LPS containing medium with fixative. We would like to acknowledge NASA and Dutch Space for the use of their illustrations in this figure.
Activation of p38 map kinase is not affected by space flight-associated microgravity
One of the most pronounced biochemical changes following LPS challenge, is the phosphorylation and thereby activation of p38 MAP kinase20. Inhibition of p38 MAP kinase protects against endotoxemia in LPS challenged healthy volunteers21, although clinical implementation of pharmacological p38 MAP kinase inhibition has not proven viable because of side effects and excessive vulnerability towards bacterial infection. Various genes, like PRKCA22, whose expression is known to be exquisitely sensitive to microgravity are known to be regulated by p38 MAP kinase23. Hence, it is conceivable that activation of p38 MAPK is impaired during microgravity. As expected, the onboard 1 g control displayed p38 MAP kinase activation (Figure 2). In contrast, activation of p38 MAP kinase was not impaired in microgravity-exposed monocytes, if anything p38 MAP kinase activation appears to be increased. Thereby suggesting that space flight-associated microgravity does not interfere with LPS-induced pro-inflammatory signal transduction per se and its effects, if present, are restricted to specific signaling pathways.
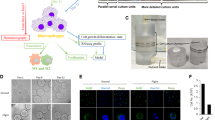
Microgravity does not influence activation of p38 MAP Kinase following LPS stimulation.
The figure shows phospho-p38 MAP kinase analysis of 100 ng/ml LPS stimulated monocytes in the presence (1 g) or relative absence of gravity (0 g). Fixed monocytes were stained fluorescently (green) and the nuclei were visualized by DAPI (blue) (Figure 2A). Staining was measured at different exposure times (Figure 2B) and mean fluorescence was finally determined at 2000 ms (Figure 2D). Although differences between the conditions are apparent, the absence of gravity does not affect activation of p38 MAP kinase. The nuclear localization of p38 MAPK is also not different from the control condition (Figure 2C). The graphs are based on 36 determinations.
Jun-n-terminal kinase is not activated by lps during space flight-associated microgravity
In general inflammatory stimuli co-activate p38 MAP kinase and Jun-N-terminal kinase, as activation of both kinases displays similar kinetics24. Nevertheless, they are activated by different upstream kinases (MKK3/6 and MKK4/7 for p38 MAP kinase and Jun-N-terminal kinase, respectively25). Thus, at least theoretically, the possibility exists that space flight-associated microgravity targets Jun-N-terminal kinase without a concomitant effect on p38 MAP kinase. Surprisingly, during microgravity the capacity of LPS to stimulate Jun-N-terminal kinase activation is lost, whereas monocytes stimulated with LPS during the onboard control condition were completely Jun-N-terminal kinase activation proficient (Figure 3). Thus, microgravity evokes a dichotomy in immunological signaling, allowing p38 MAP kinase activation to proceed, but incompatible with LPS dependent Jun-N-terminal kinase activation. Although it does not involve immunologically relevant cells, the observation that the induction of c-Jun in A431 cells by epidermal growth factor is absent in microgravity26, fits well with our findings. Especially since it has been well established that Jun-N-terminal kinase is involved in the induction of c-Jun and p38 MAPK is not.
LPS-dependent Jun-N-terminal kinase activation is sensitive to space flight-associated microgravity.
The figure shows phospho-Jnk analysis of LPS stimulated monocytes in the presence (1 G) or relative absence of gravity (0 G). Fixed monocytes were stained fluorescently (green) and the nuclei were visualized by DAPI (blue) (Figure 3A). Comparison between individual cells clearly shows a brighter staining when stimulated with LPS in the presence of gravity. Staining intensity was measured at different exposure times (Figure 3B) and mean fluorescence was finally determined at 2000 ms (Figure 3D). Microgravity significantly impairs the activation of Jnk in monocytes. Analysis of nuclear localization of activated Jnk shows no change (Figure 3C). The graphs are based on 32 determinations. *P < 0.05.
Effects of space flight-associated microgravity on erk activation
Although less pronounced than the effect on Jun-N-terminal kinase, an inhibitory influence of microgravity on the capacity of LPS to provoke activation of the extracellular regulated kinase (ERK) was observed (Figure 4). This corresponds with recent findings in T cells that show a minor effect of microgravity on ERK phosphorylation in unstimulated T cells27. Thus, the effect of microgravity is not solely restricted to Jun-N-terminal kinase.
Effects of microgravity on ERK activation.
The figure shows phospho-Erk analysis of LPS stimulated monocytes in the presence (1 G) or relative absence of gravity (0 G). Fixed monocytes were stained fluorescently (green) and the nuclei were visualized by DAPI (blue) (Figure 4A). Comparison between individual cells clearly shows a brighter staining when stimulated with LPS in the presence of gravity. Staining intensity was measured at different exposure times (Figure 4B) and mean fluorescence was finally determined at 2000 ms (Figure 4D). Microgravity impairs the activation of Erk in monocytes. Analysis of nuclear localization of activated Erk shows no change (Figure 4C). The graphs are based on 32 determinations.
Diminished activity of the immune system is among the foremost effects of space flight on the human body28. Importantly, this diminished activity of the immune system is well-tolerated. Over forty years of experience with manned space flight have now conclusively demonstrated that man can easily survive and work in weightless conditions, despite chronic repression of the body's immune functions29. This suggests that mimicking space-related immunosuppression on earth may be a fairly safe way of suppressing the action of the immune system. Although experimental requirements allowed for only 6 minutes of microgravity, there is good evidence that even a relatively short inhibition of kinases can have a substantial effect on functionality of the innate immune system30. Therefore we feel that the observed effect on Jun-N-terminal kinase is relevant to the explanation of space flight-associated immunosuppression. The observed dichotomy between p38 MAP kinase and Jun-N-terminal kinase is consistent with these observations: p38 MAP kinase is important for fighting immediate bacterial threats and thus its inhibition causes undesirable side effects in these and other respects31. On the other hand, Jun-N-terminal kinase is more associated with chronic inflammatory responses32,33 whose inhibition represents a clear and for now unresolved clinical need.
Methods
Microgravity experimentation
This study received Institutional Review Board approval from the Academic Medical Center and informed consent was obtained in accordance with the Declaration of Helsinki. The methods were carried out in accordance with the approved guidelines. Blood was drawn from young healthy volunteers (health status was ascertained by a physical examination) in Vacutainer tubes (Becton-Dickinson, Breda, the Netherlands) containing EDTA-K3. Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood of healthy volunteers using standard density gradient centrifugation over Ficoll-Paque Plus (Amersham Biosciences AB), followed by washing and resuspension in IMDM supplemented with 10% fetal bovine serum, penicillin, streptomycin and amphotericin. Blood was drawn ten hours before launch time. The experiments were performed in the “Biology In Microgravity” flight module that has been developed by Dutch Space (Leiden, the Netherlands) and the Kubik module developed by ESA (Noordwijk, The Netherlands), which were loaded with automated experimental container units developed by the Centre for Concepts of Mechatronics (Nuenen, The Netherlands). Launch vehicles were sounding rockets developed by Swedish Space Corporation (SSC) and the Soyuz-TMA rocket produced through the Russian space programme. Experiments employed a late access unit, which was installed into the payload 4 hours before launch. An integrated thermal controller maintained the experiment temperature at 36.5 ± 0.5°C. Monocytes, a 100 ng/ml LPS containing activation solution and the 4% formaldehyde fixative in PBS were loaded into the flight hardware. The hardware devices contain two experimental units of which each unit contains three chambers. The two outer chambers were connected via small tubes in the lid with the middle chamber which contained the biological material. The chambers for the fixative contain special storage compartments, since fixatives for biological material diffuse easily through rubbers and plastics. Electronically-controlled springs released activation solution and fixative at pre-set time points, so that the solutions in the storage chambers are pushed via the connecting tubes into the reaction chamber. The LPS-containing solution or the vehicle control was transferred shortly after the onset of microgravity, whereas the fixative was transferred exactly 5 min later. Technical failure was a substantial problem: of the 96 biological experiments send in space only 31 samples obtained from the Maser 12 flight qualified for further analysis, limiting the number analyses possible. Realistic vibration tests with the BIM-2 module were performed at the Environmental Test Laboratory of Thales Cryogenics (Eindhoven, The Netherlands) during flight qualification procedures and did not affect experimental outcomes (not shown). Accelerations of the payload and of the centrifuge were continuously monitored and recorded during flight.
Analysis of kinase activation
Following recovery, cells were extensively washed with PBS and permeabilized with PBS containing 0.1% Triton X-100. Subsequently, samples were incubated overnight at 4°C with anti-phospho-p38 MAPK (pThr180/pTyr182) monoclonal rabbit antibody, anti-phospho-Jun-N-terminal kinase (pThr183/pTyr185) monoclonal rabbit antibody or anti-phospho-Erk1/2 (pThr202/pTyr204) monoclonal rabbit antibody, all obtained from Cell signaling Technology (http://www.cellsignal.com/) followed by incubation with the secondary antibody anti-rabbit AlexaFluor488 (Invitrogen, Carlsbad, CA) for 1 h at room temperature and counterstained with DAPI. Confocal images were obtained on a Leica TCS SP2 system equipped with 405-nm UV, 488-nm argon and 543-HeNe lasers (Leica, Mannheim, Germany) and processed using ImageJ (National Institutes of Health, Bethesda, MD) software for constructing total fluorescence signal and automated analysis of nuclear versus cytoplasmic signal.
Statistical analysis
Tests for statistical significance of differences were made by heteroscedastic 2-tailed student t test using the program SPSS 16.0. Differences were considered significant when p < 0.05.
References
Knight, V., Couch, R. B. & Landahl, H. D. Effect of lack of gravity on airborne infection during space flight. JAMA 214, 513–518 (1970).
Cogoli, A., Tschopp, A. & Fuchs-Bislin, P. Cell sensitivity to gravity. Science. 225, 228–230 (1984).
Sonnenfeld, G. Space flight modifies T cellactivation—role of microgravity. J Leukoc Biol 92, 1125–1126 (2012).
Chang, T. T. et al. The Rel/NF-κB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J Leukoc Biol. 92, 1133–1145 (2012).
Turner, N. D., Braby, L. A., Ford, J. & Lupton, J. R. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition 18, 904–12 (2002).
Marino, A. A. et al. Nonlinear response of the immune system to power-frequency magnetic fields. Am J Physiol Regul Integr Comp Physiol. 279, R761–R768 (2002).
Salim, S., Chugh, G. & Asghar, M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 88, 1–25 (2012).
Stowe, R. P., Mehta, S. K., Ferrando, A. A., Feeback, D. L. & Pierson, D. L. Immune responses and latent herpesvirus reactivation in spaceflight. Aviat Space Environ Med 72, 884–891 (2001).
Mehta, S. K., Cohrs, R. J., Forghani, B., Zerbe, G., Gilden, D. H. & Pierson, D. L. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol 72, 174–179 (2004).
Stowe, R. P. et al. Leukocyte subsets and neutrophil function after short-term spaceflight. J Leukoc Biol. 65, 179–186 (1999).
Kaur, I., Simons, E. R., Castro, V. A., Ott, C. M. & Pierson, D. L. Changes in monocyte functions of astronauts. Brain Behav Immun. 19, 547–554 (2005).
Kaur, I., Simons, E. R., Kapadia, A. S., Ott, C. M. & Pierson, D. L. Effect of spaceflight on ability of monocytes to respond to endotoxins of gram-negative bacteria. Clin Vaccine Immunol. 15, 1523–1528 (2008).
Nichols, H. L., Zhang, N. & Wen, X. Proteomics and genomics of microgravity. Physiol Genomics. 26, 163–171 (2006).
Boonyaratanakornkit, J. B. et al. Key gravity-sensitive signaling pathways drive T cell activation. FASEB J. 19, 2020–2022 (2005).
Cohrs, R. J., Mehta, S. K., Schmid, D. S., Gilden, D. H. & Pierson, D. L. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol 80, 1116–1122 (2008).
Jin, M. S. & Lee, J. O. Structures of the toll-like receptor family and its ligand complexes. Immunity 29, 182–191 (2008).
O'Neill, L. A., Golenbock, D. & Bowie, A. G. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 13, 453–460 (2013).
Diks, S. H. et al. Kinome profiling for studying lipopolysaccharide signal transduction in human peripheral blood mononuclear cells. J Biol Chem 279, 49206–49213 (2004).
Zhou, L. et al. Impact of human granulocyte and monocyte isolation procedures on functional studies. Clin Vaccine Immunol. 19, 1065–1074 (2012).
Kasper, C. A. et al. Cell-cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity 33, 804–816 (2010).
Branger, J. et al. Inhibition of coagulation, fibrinolysis and endothelial cell activation by a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. Blood 101, 4446–4448 (2003).
Grosse, J. et al. Short-term weightlessness produced by parabolic flight maneuvers altered gene expression patterns in human endothelial cells. FASEB J. 26, 639–655 (2012).
Hsieh, Y. H. et al. p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res. 67, 4320–4327 (2007).
Gallagher, E. et al. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 8, 57–63 (2007).
Gallo, K. A. & Johnson, G. L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 3, 663–672 (2002).
de Groot, R. P. et al. Microgravity decreases c-fos induction and serum response element activity. J Cell Sci 97, 33–38 (1990).
Tauber, S. et al. Signal transduction in primary human T lymphocytes in altered gravity - results of the MASER-12 suborbital space flight mission. Cell Commun Signal. 11, 32; 10.1186/1478-811X-11-98 (2013).
Becker, J. L. & Souza, G. R. Using space-based investigations to inform cancer research on Earth. Nat Rev Cancer. 13, 315–327 (2013).
van Loon, J. J. W. A. in Biology in Space and Life on Earth. Effects of Spaceflight on Biological Systems. (ed. Brinckmann, E.) 17–32 (Wiley-VCH, 2007).
Comalada, M., Lloberas, J. & Celada, A. MKP-1: a critical phosphatase in the biology of macrophages controlling the switch between proliferation and activation. Eur J Immunol. 42, 1938–1948 (2012).
Cuadrado, A. & Nebreda, A. R. Mechanisms and functions of p38 MAPK signalling. Biochem J. 429, 403–417 (2010).
Coquet, J. M. et al. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity 38, 53–65 (2013).
Braat, H. et al. Consequence of functional Nod2 and Tlr4 mutations on gene transcription in Crohn's disease patients. J Mol Med 83, 601–609 (2005).
Acknowledgements
The authors are grateful for the assistance provided by the Netherlands Space Office (especially Rolf de Groot), ESA (especially René Demets), the Center for Concepts in Mechatronics (especially Edwin Langerak), Dutch Space and the Swedish Space Corporation (especially Per Holm) and all others who contributed to the success of this study. The authors also want to acknowledge the support of ALW (840.12.001).
Author information
Authors and Affiliations
Contributions
A.V. & E.H. – planned the experiments, performed the experiments and wrote the manuscript. A.T., W.U., J.D., E.B. – identified patients, planned the experiments and wrote the paper. V.M. & M.P. – planned the experiments and wrote the paper. All authors reviewed the final draft.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Verhaar, A., Hoekstra, E., Tjon, A. et al. Dichotomal effect of space flight-associated microgravity on stress-activated protein kinases in innate immunity. Sci Rep 4, 5468 (2014). https://doi.org/10.1038/srep05468
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05468
This article is cited by
-
Analysis of the effects of magnetic levitation to simulate microgravity environment on the Arp2/3 complex pathway in macrophage
Journal of Biological Physics (2021)
-
Microgravity induces autophagy via mitochondrial dysfunction in human Hodgkin’s lymphoma cells
Scientific Reports (2018)
-
Microgravity Level Measurement of the Beijing Drop Tower Using a Sensitive Accelerometer
Scientific Reports (2016)
-
A systems biology pipeline identifies new immune and disease related molecular signatures and networks in human cells during microgravity exposure
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.