Abstract
We report intensely staining epiretinal membrane (ERM) with Brilliant Blue G (BBG) under air for two minutes. ERM peeling was performed in 21 cases. After removal of posterior hyaloid, 0.2 mL BBG was first applied on the macula, to stain ERM under air conditions for 2 minutes. Internal limiting membrane (ILM) was intensely stained and peeled in all cases following ERM removal. In 4 cases, the ERM was also observed to be intensely stained with BBG and peeled with an ILM forceps. Postoperatively, the ganglion cell layer thickness was lower in three of the cases, however VA improved in all cases and multifocal electroretinogram revealed no toxicity. Light microscopy of ERM revealed masses of cells whereas; the ILM did not. The increased staining characteristics of ERM and ILM may be resulted from longer contact time of BBG under air pressure.
Similar content being viewed by others
Introduction
In macular surgeries, the intravitreal application of brilliant blue G (BBG) has gained popularity to visualize the internal limiting membrane (ILM). In contrast to other vital dyes such as indocyanine green (ICG), BBG was shown to have a good safety profile providing satisfactory anatomical and functional postoperative results1,2. However, the presence of epiretinal membrane (ERM) formation may impair sufficient staining and complete removal of the ILM, which may result ERM recurrences due to residual ILM fragments including indefinite extent of cells and collagen remnants at the vitreal side of the ILM3. In a previous study Shimada et al.4 found BBG has high affinity to ILM and low affinity to ERM. Additionally, Schumann et al.5 reported BBG showed no staining with ERM specimens intra-operatively. In this report, we present our observations about BBG's staining features for ERM and outcomes of the longer exposure time for BBG under air pressure using optical coherence tomography (OCT) and multifocal electroretinogram (mfERG).
Results
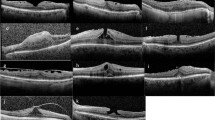
In 4 cases ERM was intensely stained with BBG. Table 1 summarizes best corrected visual acuity (BCVA) measurements and OCT results of the patients preoperatively and postoperatively at 1 month. In all cases the BCVA improved, central macular thickness (CMT) decreased, however, the macular ganglion cell–inner plexiform layer (GC-IPL) thickness decreased postoperatively in three cases. mfERG revealed no toxicity at postoperative first month (Table 2). In all cases P1 amplitude improved and the P1 implicit time reduced at the first month of the surgery (Fig. 1 right, Fig. 1 left).
Histopathologic examination of the ERM revealed fibroblast like cells along with extensive connective tissue. The ILM was absent of cells and a small amount of connective tissue was observed (Fig. 2).
Discussion
ERM recurrence is observed in approximately %10 of cases after surgery6. The reasons for recurrence are the incomplete removal of the ERM and the persisted ILM after ERM peeling, even the ERM seems to have been completely peeled3. To enhance the visualization of these transparent or semitransparent structures and to overcome ERM recurrence, various staining methods have been used, including indocyanine green (ICG), trypan blue (TB), triamcinolone acetonide (TA) and brilliant blue G (BBG)7. Of these stains, BBG shows no retinal toxicity or adverse effects related to the dye were observed in animal and human studies2.
ILM that persists after ERM peeling acts as a scaffold for cell proliferation, leading to ERM recurrence. In a previous study, Shimada et al.4 found that BBG has low affinity to ERM and recommended the reapplication of BBG after ERM peeling to remove the residual ILM to reduce the risk of ERM recurrence. Soon after Schumann et al.5 reported that BBG showed no staining with ERM specimens intra-operatively because massive epiretinal cell and collagen proliferation prevented a positive staining with BBG. They also noted that after having peeled the unstained ERM, the residual ILM was selectively stained by BBG even when a small amount of cells and collagen adheres to its vitreal side. In this study, BBG has shown low affinity to ERM in the majority of the eyes, whereas intensely stained it in 4 of 21 cases (%19).
In previous reports4,5 BBG was applied into the fluid filled vitreous cavity and washed out immediately. In the present report the intense staining of ERM with BBG may be due to longer application time of the dye under air pressure, and/or different collagen and other matrix constituents within the ERM8.
In a previous report, the ganglion cell (GCC) thickness in the postoperative period was significantly lower than preoperative, in patients underwent macular hole surgery with ICG. This result was explained with the mechanical damage to the retina by ILM peeling or cytotoxicity of ICG9. In three of our cases, we observed a decrease in GC-IPL thickness. This result may be related to two possibilities; first the mechanical damage of the surgery as mentioned in a previous study. Second but the weaker possibility of BBG toxicity on GC-IPL, which still have not affected the mfERG results and BCVA in these cases.
An objective assessment of the visual function can be made by mfERG. Multifocal ERG selects the elecrophysiological responses of multiple retinal locations of the macular and perimacular area, which are tested simultaneously, allowing functional mapping of the central retina. In our cases, mfERG functional results also showed an improvement at follow-up visit.
In conclusion it seems that the longer application time of BBG under air pressure may safely increase the ability for ERM and ILM staining. However, larger case series with longer follow-up periods are needed to confirm our findings.
Methods
21 patients with idiopathic ERMs were examined prospectively before and after vitrectomy. All surgeries were performed at the Tugut Özal University, Department of Ophthalmology between 2012 and 2013, by one experienced vitreoretinal surgeon (YT). We included eyes with idiopathic ERM with significant loss of visual acuity (20/40). Eyes with other macular pathology, history of ocular inflammation and previous ocular surgery and patients with systematic disorders affecting the eye, such as diabetes, were excluded from the study. Patients with ERM and lamellar hole were also excluded from the study. All patients were informed for the procedures of the study and gave their written consent.
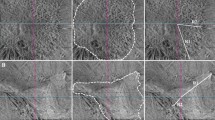
We performed a standard 23 Gauge transconjunctival sutureless vitrectomy (TSV) with EIBOS wide field imaging system under 25 Gauge Torpedo minilight illumination in 21 cases with a diagnosis of ERM based on preoprative OCT. Following core vitrectomy and removal of posterior hyaloid, we injected 0.2 mL BBG with a concentration of 0.25 mg/mL, 0.025% (Brilliant Peel; Geuder, Heidelberg, Germany) on the macula under air conditions for 2 minutes and washed out with irrigation solution. ERM was observed to be intensely stained with BBG in 4 cases and peeled easily with an internal limiting membrane (ILM) forceps (Fig. 3 left). After the ERM was peeled the ILM was again stained with BBG and peeled easily and safely in the same fashion (Fig. 3 right).
Patients were examined preoperatively and at 1 month postoperatively. Examination included slit-lamp examination, fundus examination, the best corrected visual acuity (BCVA-as the logarithm of the minimum angle of resolution (logMAR)), OCT and mfERG. For the OCT examination the Cirrus HD OCT (Carl Zeiss Meditec Inc., Dublin, California) was used to assess the central macular thickness and ganglion cell layer analyses (GCA). The prototype GCA algorithm, incorporated in Cirrus HD-OCT software Version 6, was used to process and measure the thickness of macular GC-IPL within a 14.13 mm2 elliptical annulus area centered on the fovea.
For the mfERG, the RETIscanTM (Roland Consult system, GmbH, Wiesbaden) was used. Area 1 that corresponds to the 2.8 central degrees of the retina and area 2 that extends from 2.8 to 9 degrees from the center of the fovea were evaluated for the retinal toxicity. The major component P1 amplitudes and implicit times of responses were recorded and assessed.
In a randomly selected case, the stained ERM and ILM were also examined histopathologically. ERM and ILM specimen was fixed in 10% formalin in phosphate buffer (pH 7.4), embedded in paraffin, stained with hematoxylin and eosin and then examined under a light microscope.
References
Enaida, H. et al. Preclinical investigation of internal limiting membrane staining and peeling using intravitreal brilliant blue G. Retina 26, 623–30 (2006).
Remy, M. et al. An in vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophthalmol 92, 1142–147 (2008).
Kifuku, K. et al. Residual internal limiting membrane in epiretinal membrane surgery. Br J Ophthalmol 93, 1016–019 (2009).
Shimada, H. et al. Double staining with brilliant blue G and double peeling for epiretinal membranes. Ophthalmology 116, 1370–376 (2009).
Schumann, R. G. et al. Sequential epiretinal membrane removal with internal limiting membrane peeling in brilliant blue G-assisted macular surgery. Br J Ophthalmol 94, 1369–372 (2010).
Grewing, R. & Mester, U. Results of surgery for epiretinal membranes and their recurrences. Br J Ophthalmol 80, 323–26 (1996).
Kwok, A., Lai, T. Y. & Yuen, K. S. Epiretinal membrane surgery with or without internal limiting membrane peeling. Clin Experiment Ophthalmol 33, 379–85 (2005).
Kritzenberger, M. et al. Different collagen types define two types of idiopathic epiretinal membranes. Histopathology 58, 953–65 (2011).
Baba, T. et al. Reduction of thickness of ganglion cell complex after internal limiting membrane peeling during vitrectomy for idiopathic macular hole. Eye (Lond) 26, 1173–80 (2012).
Author information
Authors and Affiliations
Contributions
Y.T. performed all ERM surgeries, E.G. helped to write the main manuscript text and M.S.D. helped to perform examinations.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Totan, Y., Güler, E. & Dervişoğulları, M. Brilliant Blue G assisted Epiretinal Membrane Surgery. Sci Rep 4, 3956 (2014). https://doi.org/10.1038/srep03956
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03956
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.