Abstract
Selective activation of primary C–H bonds for fine chemicals synthesis is of crucial importance for the sustainable exploitation of available feedstocks. Here, we report a viable strategy to synthesize ternary GR-CdS-TiO2 composites with an intimate spatial integration and sheet-like structure, which is afforded by assembling two co-catalysts, graphene and TiO2, into the semiconductor CdS matrix with specific morphology as a visible light harvester. The GR-CdS-TiO2 composites are able to serve as a highly selective visible-light-driven photocatalyst for oxidation of saturated primary C–H bonds using benign oxygen as oxidant under ambient conditions. This work demonstrates a wide, promising scope of adopting co-catalyst strategy to design more efficient semiconductor-based photocatalyst toward selective activation of C–H bonds using solar light and molecular oxygen.
Similar content being viewed by others
Introduction
Selective oxidation of saturated sp3 C–H bonds has been remaining a significant and challenging task in both fundamental and chemical industry toward the exploitation of available feedstocks in a sustainable manner1,2,3,4,5,6,7,8. However, selective oxidation of C–H bonds with environmentally benign oxygen is often very difficult to control because the activation of very inert C–H bonds typically requires harsh reaction conditions, e.g., high pressure and high temperature; with such reaction conditions, the reactivity of molecular oxygen is difficult to control, thereby resulting in poor selectivity or overoxidation. Commercial processes for selective oxidation of such C–H bonds are often based on thermocatalytic approaches involving the use of metal catalysts, high temperature and/or high pressure9. Recent years have witnessed significant progress regarding heterogeneous thermocatalytic selective oxidation of C–H bonds, which includes selective oxidation of primary C–H bonds in toluene over alloyed Au-Pd nanoparticles supported on carbon or TiO2 and metal-free g-C3N4 polymer with nanopores and selective oxidation of secondary C–H bonds in cyclohexane over metal-free nanocomposites of graphene nanosheets and C3N4 polymer9,10. Notably, the use of harsh reaction conditions, high temperature (150 ~ 160°C) and high pressure (10 bar oxygen pressure), are still required for these heterogeneous thermocatalytic selective oxidation of inert C–H bonds9,10.
Heterogeneous photocatalysis by semiconductor-based materials features the unique advantages of operation under room temperature and utilization of clean, renewable solar light as the driving force11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26. Therefore, heterogeneous photocatalysis under ambient conditions and visible light represents an attractive, promising alternative for selective activation of C–H bonds27,28,29,30. Fukuzumi and co-workers have reported selective oxidation of primary C–H bonds in p-xylene to produce p-tolualdehyde in an O2-saturated acetonitrile solvent over an Acr+-Mes@AlMCM-41 composite; and the formation of a long-lived electron-transfer (ET) state in Acr+-Mes@AlMCM-41 upon visible light irradiation affords the enhanced photocatalytic oxygenation reactivity27. Zhu's group has demonstrated aerobic selective oxidation of primary C–H bonds in toluene and its derivatives in benzotrifluoride (BTF) solvent to generate aldehydes, alcohols and acids over metal hydroxide nanoparticles grafted with alcohols under visible light irradiation, albeit the photoactivity is still low28. Free radicals formed on the surface complexes grafted onto metal hydroxides have been proposed to initiate the activation of C–H bonds in alkyl aromatics28. Similarly, Yuan and co-workers recently have shown a chlorine-radical-mediated visible-light-driven photocatalytic activation of C–H bonds in alkyl aromatics and cyclohexane in BTF solvent and even solvent-free conditions over surface-chlorinated BiOBr/TiO2 composites (CBT)29.
Very recently, our group has reported that tuning the microstructure and morphology composition in a very simple way is able to transform the well-known semiconductor CdS into an efficient visible light photocatalyst for oxidation of primary C–H bonds with dioxygen as benign oxidant30. In particular, the as-prepared cubic phase, sheet structured CdS is extremely selective for aerobic oxidation of primary C–H bonds in toluene and substituted toluenes to corresponding aldehydes. However, similar to previous research works27,28,29, the low photoactivity of CdS highlights the fact that there is still a need to improve the photocatalytic performance of CdS for selective oxidation of C–H bonds. Thus, the optimization of light capture, separation and transfer of photogenerated charge carriers over the semiconductor CdS surface is of vital importance toward the achievement of enhanced photoactivity of CdS for C–H activation under visible light. In this regard, the strategy of constructing spatial integration of semiconductor and co-catalysts to form composite materials in an appropriate manner is potentially useful to enhance the photoactivity of semiconductor CdS, because the co-catalysts are able to cooperate with the light harvester to facilitate the charge separation/transfer and lengthen the lifetime of photogenerated electron-hole pairs, thereby resulting in an increase in the overall photocatalytic performance31,32,33.
Graphene (GR), a single layer of graphite, has superior electron conductivity/mobility and been shown to be an efficient co-catalyst to assemble with semiconductors to form graphene-semiconductor composite photocatalysts, which can exhibit enhanced photoactivity in solar energy conversion, e.g., photocatalytic pollutants degradation, water splitting, CO2 reduction and selective oxidation of alcohols20,21,34. On the other hand, the intrinsic energy band structure match between CdS and wide-band-gap TiO2 indicates the feasibility of directional migration of photogenerated electrons from CdS to the conduction band (CB) of TiO2 under visible light irradiation35. Thus, in principle, if CdS is hybridized with two co-catalysts of graphene and TiO2 in a proper manner, the lifetime and transfer of photogenerated charge carriers, i.e., electron-hole pairs, would be efficiently improved. Consequently, it can be expected that the photoactivity of CdS toward C–H activation would be enhanced effectively. Toward this purpose, we herein demonstrate a viable strategy of assembling two co-catalysts, graphene and TiO2, with visible-light-active semiconductor CdS toward an improved photoactivity for aerobic selective C–H activation under mild conditions, room temperature and atmospheric pressure.
Results
We initially have prepared GR-CdS composites aiming to (i) determine the optimal ratio of GR in order to get an optimal enhancement of CdS photoactivity toward aerobic C–H oxidation via the addition of GR as a co-catalyst (for synthesis details, see Supplementary Information) and (ii) provide a basis for further constructing ternary GR-CdS-TiO2 composites as discussed later. The CdS sample was synthesized by a very simple wet chemistry method at room temperature30. As shown in Figure S1 (Supplementary Information), all the GR-CdS composites with different weight addition ratios of GR, i.e., 1, 5, 10 and 30% GR-CdS, show similar XRD patterns with that of blank-CdS sample. The peaks of scattering angles 2θ values located at ca. 26.5°, 43.9° and 52.1° are attributed to the diffractions of the (111), (220) and (311) facet crystal planes of cubic phase CdS, respectively30. No apparent peak for the separate GR in GR-CdS composites is detected in XRD patterns, which is probably because of the relative low intensity of GR at ca. 25.0° shielded by the CdS diffraction peak20,21.
The addition of GR into CdS matrix induces a significant change on the optical property of semiconductor CdS, as displayed in Figure S2 (Supplementary Information). With the increase of GR content, there is an enhanced absorbance in the visible light region ranging from 600 to 800 nm. It is noted that, compared with blank-CdS, the GR-CdS composites show the band gap narrowing trend, i.e., the red shift to higher wavelength, which is evidenced by the plot of transformed Kubelka-Munk function versus the energy of light (Figure S2b). The band gaps of blank-CdS, 1%GR-CdS, 5%GR-CdS, 10%GR-CdS and 30% GR-CdS are estimated to be ca. 2.27 eV, 2.27 eV, 2.26 eV, 2.15 eV and 2.10 eV, respectively, indicating a band gap narrowing of semiconductor CdS after its hybridization with GR.
The photoactivity of activation of C–H bond in toluene over GR-CdS composites has been tested using the same reaction condition as that over blank-CdS photocatalyst under visible light irradiation. It can be seen from Figure 1 that, under visible light irradiation of 10 h, 53% conversion of toluene is achieved over the optimal 5%GR-CdS composite, which is much higher than 33% conversion over the blank-CdS photocatalyst under identical reaction conditions. On the other hand, the selectivity to benzaldehyde can still be maintained as high as 99%, indicating that the proper amount addition of GR not only improves the photoactivity of CdS for C–H activation in toluene, but also does not play a significant effect on the achievement of high selectivity to benzaldehyde. The excessive addition of GR, e.g., the 30%GR-CdS sample, will dramatically lower the photoactivity of CdS while the selectivity is decreased moderately. This is mainly because that the higher addition of GR lowers the contact surface of CdS with the light illumination and efficiency of light passing through the depth of the reaction solution, also called a “shielding effect” as widely observed in GR-semiconductor composite photocatalysts20,21,34. In the next, when we introduce the second co-catalyst TiO2 to further improve the photoactivity of CdS catalyst toward C–H activation, the addition ratio of co-catalyst GR is set as 5% to construct ternary GR-CdS-TiO2 composites (details for synthesis of GR-CdS-TiO2 in the Supplementary Information).
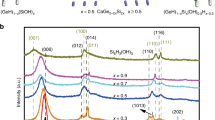
Figure 2a shows the XRD patterns of ternary 5%GR-CdS-TiO2 composites with different weight addition ratios of TiO2. It is seen that, when the weight addition ratio of TiO2 is low, i.e., 1, 5 and 10% TiO2, the 5%GR-CdS-TiO2 composites show similar XRD patterns with that for 5%GR-CdS. When the weight ratio of TiO2 is increased to 30 and 50%, the characteristic diffraction peaks at 25.3°, 37.8° and 48.0° are clearly observed, which are attributed to the (101), (004) and (200) facets of anatase TiO235. According to the calculation based on the Scherrer formula, the average crystallite sizes of CdS and TiO2 in the GR-CdS-TiO2 composite are determined to be about 1.9 nm and 5.0 nm, respectively. As expected, with the addition of second co-catalyst TiO2, the photoactivity toward aerobic oxidation of C–H in toluene is further enhanced as compared to the binary 5%GR-CdS composite, which is evidenced by the photoactive test in Figure 2b. For example, 69% conversion of toluene can be obtained over the optimal ternary 5%GR-CdS-10%TiO2 composite under visible light irradiation, which is higher than 53% and 33% conversion for that over 5%GR-CdS and blank-CdS photocatalyst, respectively. In addition, to evaluate the stability of photocatalyst, we have performed recycling photoactivity test for the 5%GR-CdS and 5%GR-CdS-10%TiO2 composites. As shown in Figure S3 (Supplementary Information), after 5 times recycling test, no significant loss of photoactivity is observed, because the conversion of toluene over 5%GR-CdS and 5%GR-CdS-10%TiO2 is similar to the fresh samples while the selectivity to benzaldehyde is maintained > 97%. Therefore, the as-prepared composite samples are stable visible-light-driven photocatalyst for the selective oxidation of toluene in the reaction medium of BTF solvent under ambient conditions. Furthermore, we have also carried out the reaction for oxidation of toluene to benzaldehyde over 5%GR-CdS-10%TiO2 by extending the reaction time, which is shown in Figure S4 (Supplementary Information). It can be seen that the conversion of toluene is enhanced with the reaction time is progressing. After visible light irradiation of 19 h, the conversion of toluene over 5%GR-CdS-10%TiO2 is up to 98% while the selectivity to benzaldehye is 95%. In addition, to ensure if this photoactivity enhancement is general, we have performed selective oxidation of other substituted toluenes over these photocatalysts under visible light irradiation. As shown in Table 1, the optimal ternary 5%GR-CdS-10%TiO2 shows higher photoactivity than both 5%GR-CdS and blank-CdS toward selective oxidation of substituted toluenes to corresponding aldehydes with high selectivity > 98%. Considering the fact that GR, blank-TiO2 or 5%GR-TiO2 almost does not have the photoactivity for toluene conversion under identical reaction conditions, as shown in Figure S5 (Supplementary Information), it can be concluded that the primary photoactive ingredient in ternary 5%GR-CdS-10%TiO2 composite is visible-light-responsive CdS and that the role of GR and TiO2 acts as co-catalysts to promote the photoactivity enhancement of CdS toward C–H activation in toluene and substituted toluenes. In other words, if these individual components are integrated in a rational manner, these two co-catalysts GR and TiO2 in the photocatalyst GR-CdS-TiO2 would cooperate with the light harvester (semiconductor CdS) to facilitate the charge carriers separation/transfer and lengthen the lifetime of photogenerated electron-hole pairs from band-gap-photoexcitation of CdS under visible light irradiation, thereby resulting in an increase in the overall photocatalytic performance. This inference has been faithfully verified by the comparative analysis of photoelectrochemical and photoluminescence (PL) spectra on the optimal 5%GR-CdS-10%TiO2, 5% GR-CdS and blank-CdS. In the following discussion section, we will show that the enhanced photoactivity is primarily attributed to the improved lifetime and transfer of photogenerated charge carriers. In addition, the possible reaction mechanism for selective oxidation of toluene over the optimal 5%GR-CdS-10%TiO2 under visible light irradiation is also studied and discussed.
Discussion
As shown in Figure 3a, under visible light irradiation, the photocurrent transient response for 5%GR-CdS is higher than blank-CdS, indicating that the addition of GR as co-catalyst into the semiconductor CdS matrix can improve the lifetime of photogenerated charge carriers. With regard to the optimal 5%GR-CdS-10%TiO2 under visible light irradiation, the photocurrent transient response is the highest, suggesting that the addition of second co-catalyst TiO2 is able to further improve the lifetime of photogenerated carriers. Figure 3b shows the electrochemical impedance spectroscopy (EIS) Nyquist plots of the electrode samples of 5%GR-CdS-10%TiO2, 5%GR-CdS and blank-CdS under visible light irradiation. It is clearly seen that, with the addition of GR and TiO2, the semi-circle in the plot becomes shorter, which indicates a decrease in the solid state interface layer resistance and the charge transfer resistance on the surface36. Thus, the charge carriers transfer over 5%GR-CdS-10%TiO2 is more efficient than that over 5%GR-CdS and blank-CdS. The PL spectra analysis (Figure S6, Supplementary Information) further shows that, after the coupling of CdS with two co-catalysts GR and TiO2, the quenching degree of PL intensity is obviously enhanced, suggesting that the lifetime of photogenerated charge carriers over the ternary 5%GR-CdS-10%TiO2 is the highest among these three samples37. Since the conduction band of CdS is more negative than that of TiO2 or graphene, the transfer of photogenerated electrons from band-gap-photoexcitation of CdS under visible light irradiation to the conduction band of TiO2 or graphene is energetically favorable, which thus improves the fate and transfer of charge carriers in the ternary 5%GR-CdS-10%TiO2 system21,35. These consequently contribute to the highest photoactivity of 5%GR-CdS-10%TiO2 toward activation of C–H bonds in toluene and substituted toluenes under visible light irradiation.
To further understand the electron transfer, we have performed the Mott-Schottky plots for GR, blank-TiO2 and blank-CdS, as displayed in Figure S7 (Supplementary Information). The Fermi level (EF) of GR and flat band potential (EFB) of TiO2 and CdS, as calculated from the X intercepts of the linear region, are found to be −0.30 V, −0.50 V and −0.71 V versus Ag/AgCl (equivalent to −0.10 V, −0.30 V and −0.51 V versus normal hydrogen electrode, NHE), respectively. Because of EF (GR) > ECB (TiO2) > ECB (CdS), electrons will transfer from the conduction band (CB) of CdS to TiO2 and then from TiO2 to GR, thus forming a long-lived electron-transfer state, which is beneficial for the overall photoactivity enhancement and in line with the above PL analysis and photoactivity data.
To learn the microscopic structure and morphology, we have performed the TEM analysis on the optimal ternary 5%GR-CdS-10%TiO2 composite and the results are displayed in Figure 4. It can be clearly seen that the two co-catalysts GR and TiO2 are intimately integrated with the semiconductor CdS matrix to form the ternary GR-CdS-TiO2 composite with a large sheet structure. Such intimate interfacial contact has been well recognized to be a beneficial factor for the charge carriers transfer across the interface20,21. The stacking GR layers can be distinctly identified from the high-resolution TEM (HRTEM) image, as displayed in panel b in Figure 4. In addition, two main distinct lattice fringes are clearly distinguished in the HRTEM image. That is, the spacing of 0.336 and 0.352 nm can be ascribed to the (111) and (101) crystal face of cubic phase CdS and anatase TiO2, respectively. The elemental mapping analysis corresponding to the green line boxed area in panel a of Figure 4 further confirms the coexistence of CdS, TiO2 and GR, which are evenly distributed in the GR-CdS-TiO2 composite. In addition, the energy dispersive X-ray (EDX) analysis also confirms the coexistence of CdS, TiO2 and GR (Figure S8, supplementary information).
To further ensure that the lifetime of photogenerated charge carriers is the most predominant factor determining the overall photocatalytic performance of GR-CdS-TiO2 toward selective oxidation of C–H bond in toluene, we have performed the specific surface area analysis and the results are listed in Table S1. The surface area of blank-CdS, 5%GR-CdS and 5%GR-CdS-10%TiO2 is ca. 132 m2·g−1, 128 m2·g−1 and 139 m2·g−1, respectively. The adsorption experiment for toluene in the dark also suggests that there is almost no difference in the adsorptivity of these three samples toward toluene (Figure S9, Supplementary Information). These results suggest that the surface area is not the primary factor leading to the large discrepancy in their photoactivities. On the contrary, the lifetime and transfer efficiency of photogenerated electron-hole pairs are the key and predominant factors that determine the overall photocatalytic activity of 5% GR-CdS-10%TiO2.
To understand the role of photogenerated radical species and underlying reaction mechanism involved for photocatalytic selective oxidation of toluene over the 5%GR-CdS-10%TiO2 composite under visible light irradiation, we have performed a series of control experiments in the inert N2 atmosphere and with adding different scavengers for quenching hydroxyl radicals (·OH), superoxide radicals (O2·−), holes (h+) and electrons (e−), respectively38,39. As shown in Figure 5, control experiment in N2 atmosphere shows only trace conversion of toluene, confirming that oxygen in the reaction system is the primary oxidant for oxidation of toluene. When ammonium oxalate (AO) scavenger for holes is added, the conversion of toluene is almost terminated under visible light irradiation. When benzoquinone (BQ) scavenger for superoxide radicals is added into the reaction system, the conversion of toluene is significantly inhibited. Such a remarkable inhibition on toluene conversion is also observed when K2S2O8 scavenger for electrons is added into the reaction system. Although electrons are not able to directly participate in oxidation of toluene, the electrons quenching by the addition of K2S2O8 scavenger will prevent the activation of molecular oxygen; for example, the superoxide radicals are generated from accepting photogenerated electrons by molecular oxygen. Thus, it is understandable to observe the significant inhibition of toluene conversion for both the cases of adding BQ and K2S2O8, respectively. The above control experiments clearly suggest that the positively charged holes play the predominant role in photocatalytic oxidation of toluene over 5%GR-CdS-10%TiO2. The role of negatively charged electrons is to activate molecular oxygen (e.g., the formation of superoxide radicals), which then acts as the primary oxidant for toluene conversion. In addition, it is worth noting that the addition of tert-butyl alcohol (TBA) scavenger for hydroxyl radicals plays a negligible effect on toluene conversion, which is in faithful accordance with the absence of hydroxyl radicals in the benzotrifluoride (BTF) solvent and also confirmed by our ESR analysis (Figure S10, Supplementary Information)20,23.
Control experiments of photocatalytic selective oxidation of toluene in the inert N2 atmosphere or with the addition of different radical scavengers: benzoquinone (BQ, scavenger for superoxide radicals), tert-butyl alcohol (TBA, scavenger for hydroxyl radicals), ammonium oxalate (AO, scavenger for holes) and K2S2O8 (scavenger for electrons) over the optimum 5%GR-CdS-10%TiO2 under visible light irradiation for 10 h.
Based on the above discussions, a tentative photocatalytic reaction mechanism for oxidation of toluene over the 5%GR-CdS-10%TiO2 composite can be proposed as the following. Under visible light irradiation, the electron-hole pairs are generated from CdS. The adsorbed toluene over the surface of 5%GR-CdS-10%TiO2 is able to be oxidized to the corresponding cationic radicals by the positive holes with strong oxidation power30. The photogenerated electrons from conduction band (CB) of CdS can transfer to TiO2 and GR, thus forming a long-lived electron-transfer state. Meanwhile, the electrons can react with the adsorbed O2 to give activated oxygen species (e.g., O2·−). The oxygen or activated oxygen species are then able to selectively oxidize the above cationic radicals, giving rise to the formation of the target product, benzaldehyde.
In summary, we have demonstrated a viable strategy of assembling two co-catalysts, GR and TiO2, into the semiconductor CdS matrix to form ternary GR-CdS-TiO2 composites with intimate spatial integration and sheet-like structure. The introduction of dual co-catalysts into the CdS matrix can optimize and improve the separation and transfer of charge carriers over the light harvester CdS under visible light irradiation, by which the visible light photoactivity of CdS for aerobic C–H activation under mild conditions is obviously enhanced. This work would inspire growing interest in rationally adopting co-catalyst strategy to design more efficient semiconductor-based photocatalyst toward activation of C–H bonds using solar light and benign molecular oxygen. We also expect that further optimization of the chemical compositions and synergetic interaction of GR, CdS and TiO2 may further improve the photoactivity for C–H activation in the future.
Methods
Materials
All chemicals used in this work were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and Alfa Aesar. Details were provided in the supplementary information.
Synthesis
Synthesis of graphene oxide (GO)
Graphene oxide (GO), the precursor of graphene (GR) in this work, was prepared by a modified Hummers method, which was also used in our previous studies20,21.
Synthesis of CdS sample
Our CdS sample was prepared by a very simple room temperature method, as reported earlier from our group30. Typically, 200 mL of 6.0 mM Na2S was slowly added into 200 mL of 5.0 mM Cd(Ac)2 in a drop-by-drop process for 4 h and was aged for 36 h with vigorous stirring. Then, the products were separated by filtration, washed by water and fully dried at 333 K in oven to get the final CdS sample, which is further used to construct the binary GR-CdS and ternary GR-CdS-TiO2 nanocomposites.
Synthesis of TiO2 colloids
1.32 mL of TBOT was dispersed into 30 mL C2H5OH and airproof was aged for 48 h with vigorous stirring to obtain the TiO2 colloids, which were used for construct the ternary GR-CdS-TiO2 nanocomposites.
Synthesis of GR-CdS nanocomposites
Typically, GO was ultrasonicated in 100 mL of deionized water to disperse it well and then 0.2 g of the as-prepared CdS sample was added to the given amount of the above GO solution to prepare GO-CdS nanocomposites with different weight addition ratios of GO. The mixing solution was aged with vigorous stirring for 24 h to obtain a homogeneous suspension. Then, this suspension was evaporated at water-bath 323 K in vacuum rotary evaporator and fully dried at 333 K in oven. Then, GO-CdS was aged in 40 mL of deionized water with vigorous stirring for 30 min to obtain a homogeneous suspension. After that, it was transferred to 50 mL Teflon-sealed autoclave and maintained at 393 K for 12 h, during which GO was reduced to GR20,21. Subsequently, the products were cooled to room temperature and recovered by filtration, washed by water and fully dried at 333 K in oven to get the final GR-CdS nanocomposites with different weight addition ratios of GR, namely 1, 5, 10 and 30% GR-CdS.
Synthesis of GR-CdS-TiO2 nanocomposites
Typically, GO was ultrasonicated in 100 mL of deionized water to disperse it well and then the as-prepared CdS sample and TiO2 colloids were added to the above GO solution. The mixing solution was aged with vigorous stirring for 24 h to obtain a homogeneous suspension. Then, this suspension was evaporated at water-bath 323 K in vacuum rotary evaporator and fully dried at 333 K in oven. Then, this GO-CdS-TiO2 sample was aged in 40 mL of deionized water with vigorous stirring for 30 min to obtain a homogeneous suspension. After that, it was transferred to 50 mL Teflon-sealed autoclave and maintained at 393 K for 12 h during which GO was reduced to GR20,21. After that, the products were cooled to room temperature and recovered by filtration, washed by water and fully dried at 333 K in oven to get the final GR-CdS-TiO2 nanocomposites with different weight addition ratios of TiO2. Following these steps, a series of GR-CdS-TiO2 nanocomposites, including 1, 5, 10, 30 and 50% TiO2-5% GR-CdS were obtained.
Characterization
The optical properties were analyzed by UV-vis diffuse reflectance spectroscopy (DRS) using a UV-vis spectrophotometer (Cary 500, Varian), in which BaSO4 was employed as the internal reflectance standard. The phase composition of the samples was determined by X-ray diffraction (XRD, Philip X′ Pert Pro MPP) using a Cu Kα radiation (λ = 1.5418 Å) at the scan rate of 0.08/s. The photoluminescence (PL) spectra for solid samples were investigated on an Edinburgh FL/FS900 spectrophotometer. The Brunauer-Emmett-Teller (BET) specific surface area was analyzed by nitrogen adsorption in a Micromeritics ASAP 2020 apparatus. The morphology and microscopic structure information was determined by transmission electron microscopy (TEM, FEI Tecnai G2 F20 S-TWIN). For the TEM analysis, the samples were dispersed in ethanol by ultrasonic treatment and dropped on the lacey support film without carbon coating copper grids. The radical species were detected by electron spin resonance (ESR) spectrometer on a Bruker EPR A300 instrument. The parameters for the ESR spectrometer were as follows: center field = 3507 G, microwave frequency = 9.84 GHz and power = 6.36 mW. The electrochemical analysis was carried out in a conventional three-electrode cell using a Pt plate and an Ag/AgCl electrode as the counter electrode and reference electrode, respectively. The electrolyte was 0.2 M Na2SO4 aqueous solution without additive (pH = 6.8). The photocurrent measurements were taken on a BAS Epsilon workstation without bias. The electrochemical impedance spectroscopy (EIS) were conducted on a Precision PARC workstation.
Photocatalytic activity test
Photocatalytic selective oxidation of toluene and substituted toluenes was performed under ambient conditions, i.e., room temperature and atmospheric pressure23,24,30. Typically, toluene (0.1 mmol) and 8 mg of the catalyst were dissolved in the solvent of benzotrifluoride (BTF, supplied from Alfa Aesar with a purity of > 99%) (1.5 mL) saturated with pure molecular oxygen from a gas cylinder. Such a standard reaction condition has previously been used for photocatalytic oxidation of alcohols or amines over TiO2-based semiconductor and graphene-TiO2 composites20,23,24,40. The mixture was transferred into a 10 mL Pyrex glass bottle filled with molecular oxygen at a pressure of 0.1 MPa and stirred for half an hour to make the catalyst blend evenly in the solution. The suspensions were irradiated by a 300 W Xe arc lamp (PLS-SXE 300, Beijing Perfectlight Co. Ltd.) with a UV-CUT filter (λ > 420 nm). After the reaction, the mixture was centrifuged to completely remove the catalyst particles. The remaining solution was analyzed with an Aglient Gas Chromatograph (GC-7820, with a capillary FFAP analysis column). The assignment of products was confirmed by a Hewlett-Packard gas chromatograph/mass spectrometer (HP-5973GC/MS). Controlled photoactivity experiments using different radical scavengers (ammonium oxalate as scavenger for photogenerated holes, K2S2O8 as scavenger for electrons, tert-butyl alcohol as scavenger for hydroxyl radicals and benzoquinone as scavenger for superoxide radical species) were performed similar to the above photocatalytic oxidation of toluene except that the radical scavengers (0.1 mmol) were added to the reaction system30,38,39.
References
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
bin Saiman, M. I. et al. Involvement of surface-bound radicals in the oxidation of toluene using supported Au-Pd nanoparticles. Angew. Chem. Int. Ed. 51, 5981–5985 (2012).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Sheldon, R. A. & van Bekkum, H. Fine chemicals through heterogeneous Catalysis [Sheldon, R. A. & van Bekkum, H. (ed.)] [1–10] (Wiley-VCH, Weinheim, 2001).
Kesavan, V., Sivanand, P. S., Chandrasekaran, S., Koltypin, Y. & Gedanken, A. Catalytic aerobic oxidation of cycloalkanes with nanostructured amorphous metals and alloys. Angew. Chem. Int. Ed. 38, 3521–3523 (1999).
Thomas, J. M., Raja, R., Sankar, G. & Bell, R. G. Molecular-sieve catalysts for the selective oxidation of linear alkanes by molecular oxygen. Nature 398, 227–230 (1999).
Shilov, A. E. & Shul'pin, G. B. Activation of C–H bonds by metal complexes. Chem. Rev. 97, 2879–2932 (1997).
Kesavan, L. et al. Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles. Science 331, 195–199 (2011).
Li, X. H., Chen, J. S., Wang, X., Sun, J. & Antonietti, M. Metal-free activation of dioxygen by graphene/g-C3N4 nanocomposites: functional dyads for selective oxidation of saturated hydrocarbons. J. Am. Chem. Soc. 133, 8074–8077 (2011).
Maldotti, A., Molinari, A. & Amadelli, R. Photocatalysis with organized systems for the oxofunctionalization of hydrocarbons by O2 . Chem. Rev. 102, 3811–3836 (2002).
Maeda, K., Lu, D. & Domen, K. Oxidation of water under visible-light irradiation over modified BaTaO2N photocatalysts promoted by tungsten species. Angew. Chem. Int. Ed. 52, 6488–6491 (2013).
Maeda, K. & Domen, K. Water oxidation using a particulate BaZrO3-BaTaO2N solid-solution photocatalyst that operates under a wide range of visible light. Angew. Chem. Int. Ed. 51, 9865–9869 (2012).
Tada, H., Kiyonaga, T. & Naya, S. Rational design and applications of highly efficient reaction systems photocatalyzed by noble metal nanoparticle-loaded titanium (IV) dioxide. Chem. Soc. Rev. 38, 1849–1858 (2009).
Yurdakal, S., Palmisano, G., Loddo, V., Augugliaro, V. & Palmisano, L. Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J. Am. Chem. Soc. 130, 1568–1569 (2008).
Palmisano, G. et al. Advances in selective conversion by heterogeneous photocatalysis. Chem. Commun. 46, 7074–7089 (2010).
Bard, A. J. & Fox, M. A. Artificial photosynthesis: solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 28, 141–145 (1995).
Fox, M. A. Organic heterogeneous phtocatalysis: chemical conversion sensitized by irradiation semiconductors. Acc. Chem. Res. 16, 314–321 (1983).
Shiraishi, Y. & Hirai, T. Selective organic transformations on titanium oxide-based photocatalysts. J. Photochem. Photobiol., C 9, 157–170 (2008).
Zhang, Y., Tang, Z.-R., Fu, X. & Xu, Y.-J. Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: what advantage does graphene have over its forebear carbon nanotube? ACS Nano 5, 7426–7435 (2011).
Zhang, N., Zhang, Y. & Xu, Y.-J. Recent progress on graphene-based photocatalysts: current status and future perspectives. Nanoscale 4, 5792–5813 (2012).
Zhang, N. & Xu, Y.-J. Aggregation- and leaching-resistant, reusable and multifunctional Pd@CeO2 as a robust nanocatalyst achieved by a hollow core-shell strategy. Chem. Mater. 25, 1979–1988 (2013).
Zhang, M., Chen, C., Ma, W. & Zhao, J. Visible-light-induced aerobic oxidation of alcohols in a coupled photocatalytic system of dye-sensitized TiO2 and TEMPO. Angew. Chem., Int. Ed. 407, 9730–9733 (2008).
Zhang, M. et al. Oxygen atom transfer in the photocatalytic oxidation of alcohols by TiO2: Oxygen Isotope Studies. Angew. Chem., Int. Ed. 48, 6081–6084 (2009).
Wang, Q., Zhang, M., Chen, C., Ma, W. & Zhao, J. Photocatalytic aerobic oxidation of alcohols on TiO2: the acceleration effect of a bronsted acid. Angew. Chem. Int. Ed. 49, 7976–7979 (2010).
Lang, X., Ji, H., Chen, C., Ma, W. & Zhao, J. Selective formation of imines by aerobic photocatalytic oxidation of amines on TiO2 . Angew. Chem., Int. Ed. 50, 3934–3937 (2011).
Fukuzumi, S. et al. Formation of a long-lived electron-transfer state in mesoporous silica-alumina composites enhances photocatalytic oxygenation reactivity. Proc. Natl. Acad. Sci. USA 109, 15572–15577 (2012).
Sarina, S. et al. Driving selective aerobic oxidation of alkyl aromatics by sunlight on alcohol grafted metal hydroxides. Chem. Sci. 3, 2138–2146 (2012).
Yuan, R. et al. Chlorine-radical-mediated photocatalytic activation of C–H bonds with visible light. Angew. Chem. Int. Ed. 125, 1069–1073 (2013).
Zhang, Y., Zhang, N., Tang, Z.-R. & Xu, Y.-J. Transforming CdS into an efficient visible light photocatalyst for selective oxidation of saturated primary C–H bonds under ambient conditions. Chem. Sci. 3, 2812–2822 (2012).
Zhai, Q. et al. Photocatalytic conversion of carbon dioxide with water into methane: platinum and copper(I) oxide co-catalysts with a core-shell structure. Angew. Chem. Int. Ed. 52, 5776–5779 (2013).
Fan, W., Zhang, Q. & Wang, Y. Semiconductor-based nanocomposites for photocatalytic H2 production and CO2 conversion. Phys. Chem. Chem. Phys. 15, 2632–2649 (2013).
Maeda, K. et al. Photocatalytic overall splitting promoted by two different cocatalysts for hydrogen and oxygen evolution under visible light. Angew. Chem. Int. Ed. 49, 4096–4099 (2010).
Xiang, Q., Yu, J. & Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 41, 782–796 (2012).
Liu, S., Zhang, N., Tang, Z.-R. & Xu, Y.-J. Synthesis of one-dimensional CdS@TiO2 core-shell nanocomposites photocatalyst for selective redox. The dual role of TiO2 shell. ACS Appl. Mater. Interfaces 4, 6378–6385 (2012).
Wang, D. et al. Self-assembled TiO2-graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 3, 907–914 (2009).
Xu, Y.-J., Zhuang, Y. & Fu, X. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: A case study on degradation of benzene and methyl orange. J. Phys. Chem. C 114, 2669–2676 (2010).
Zhang, Y., Chen, Z., Liu, S. & Xu, Y.-J. Size effect induced activity enhancement and anti-photocorrosion of reduced graphene oxide/ZnO composites for degradation of organic dyes and reduction of Cr(VI) in water. Appl. Catal. B 140–141, 598–607 (2013).
Zhang, Y., Zhang, N., Tang, Z.-R. & Xu, Y.-J. Identification of Bi2WO6 as a highly selective visible-light photocatalyst toward oxidation of glycerol to dihydroxyacetone in Water. Chem. Sci. 4, 1820–1824 (2013).
Lang, X., Ji, H., Chen, C., Ma, W. & Zhao, J. Selective formation of imines by aerobic photocatalytic oxidation of amines on TiO2 . Angew. Chem., Int. Ed. 50, 3934–3937 (2011).
Acknowledgements
The support by the NSFC (20903022, 20903023, 21173045), the Award Program for Minjiang Scholar Professorship and the NSF of Fujian Province for Distinguished Young Investigator Grant (2012J06003) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
M.-Q.Y., Y.Z. and N.Z. conducted the experiments. Z.-R.T. analyzed the data and discussed the results. Y.-J.X. wrote the whole manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Yang, MQ., Zhang, Y., Zhang, N. et al. Visible-Light-Driven Oxidation of Primary C–H Bonds over CdS with Dual Co-catalysts Graphene and TiO2. Sci Rep 3, 3314 (2013). https://doi.org/10.1038/srep03314
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03314
This article is cited by
-
An experimental design study of photocatalytic activity of the Z-scheme silver iodide/tungstate binary nano photocatalyst
Environmental Science and Pollution Research (2023)
-
Suzuki cross-coupling reactions over engineered AuPd alloy nanoparticles by recycling scattered light
Nano Research (2022)
-
Enhanced photocatalytic activity and charge carrier separation of CNT/TiO2/WO3/CdS catalyst for the visible-light photodegradation of reactive blue 19
Environmental Science and Pollution Research (2022)
-
Simultaneously Enhanced Activity and Selectivity for C(sp3)–H Bond Oxidation Under Visible Light by Nitrogen Doping
Transactions of Tianjin University (2021)
-
Chlorophyll-sensitized phenolic resins for the photocatalytic degradation of methylene blue and synthetic blue wastewater
Journal of Sol-Gel Science and Technology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.