Abstract
Hypoxia, a common environmental condition, influences cell signals and functions. Here, we compared the effects of hypoxia (1% oxygen) and normoxia (air) on chondrogenic differentiation of human mesenchymal stem cells (MSCs). For in vitro chondrogenic differentiation, MSCs were concentrated to form pellets and subjected to conditions appropriate for chondrogenic differentiation under normoxia and hypoxia, followed by the analysis for the expression of genes and proteins of chondrogenesis and endochondral ossification. MSCs induced for differentiation under hypoxia increased in chondrogenesis, but decreased in endochondral ossification compared to those under normoxia. MSCs induced for differentiation were more resistant to apoptosis under hypoxia compared to those under normoxia. The hypoxia-dependent protection of MSCs from chondrogenesis-induced apoptosis correlated with an increase in the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/FoxO pathway. These results suggest that the PI3K/Akt/FoxO survival pathway activated by hypoxia in MSCs enhances chondrogenesis and plays an important role in preventing endochondral ossification.
Similar content being viewed by others
Introduction
Adult cartilage tissue is avascular in nature. Moreover, the microenvironment of cartilage is hypoxic with oxygen tension as low as 1%. Therefore, chondrocytes are exposed to severe hypoxia in vivo1,2. Cartilage damaged by trauma or degenerative process has a limited capacity for self-repair. Treatment of focal or diffused cartilage defects is still a difficult and challenging problem for orthopedic surgeons3. Tissue engineering of cartilage has great potential to ameliorate cartilage-related diseases such as arthritis. Cartilage engineering is considered to offer advantages over autologous chondrocyte transplantation that is hampered by limited sources of chondrocyte and high donor site morbidity4.
Human mesenchymal stem cells (MSCs) are present in bone marrow. These multipotent cells can differentiate into chondrocytes, osteoblasts and adipocytes5. Since MSCs are a source of cells for developing novel engineered tissue constructs for treatment, of musculoskeletal diseases, such as cartilage defects, their manipulation in vitro has received significant attention in the past decade5. Chondrogenic differentiation of MSCs may be induced by specific cytokines and growth factors6, biophysical stimulation7 and provision of a suitable 3-dimensional (3-D) environment8. However, the factors that influence optimal MSC chondrogenesis remain to be fully elucidated9.
Chondrogenesis can be enhanced under hypoxic conditions10. Although, hypoxia-inducible factor-1α (HIF-1α) is the key mediator of the beneficial effect of a low oxygen environment on chondrogenesis11, the underlying mechanisms that hypoxic conditions mediate still remain unclear. It has been reported that rat pheochromocytoma PC12 cells cultured under hypoxic conditions are resistant to apoptosis induced by serum withdrawal and chemotherapy treatment12. The hypoxia-dependent protection from apoptosis correlates with activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is detected several hours after exposure to hypoxic conditions and is sustained while hypoxic conditions are maintained.
During in vivo chondrogenesis, the terminal stage, endochondral ossification, involves an ordered progression from cell division through hypertrophic differentiation to cell death. Similar to in vivo chondrogenesis, we have demonstrated that MSCs undergoing in vitro chondrogenesis express markers of endochondral ossification, such as type X collagen and Runx2 and many MSCs succumb to apoptosis13. Interestingly, PI3K/Akt signaling is also a key regulator in terminal chondrocyte differentiatio14. Activation of Akt signaling enhanced chondrocyte proliferation and inhibited hypertrophic differentiation in chondrogenesis of embryonic limb bud cells and adult synovial stromal cells, presumably due to the suppressed expression of Runx2, a transcription factor critical for chondrocyte terminal differentiation. Conversely, inhibition of PI3K by its inhibitor accelerated terminal hypertrophic differentiation.We therefore hypothesized that in vitro hypoxic culture would decrease apoptosis and thereby prevent terminal differentiation of MSCs undergoing chondrogenesis. The detailed mechanism underlying hypoxia-mediated inhibition of apoptosis was well characterized in the current study.
Results
Hypoxic culture enhanced chondrogenesis and suppressed the expression of markers of endochondral ossification in MSCs
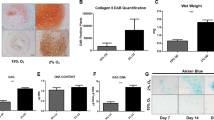
To determine the effects of hypoxic culture on MSC chondrogenesis, we first examined the mRNA levels of Sox9, Col2a1, aggrecan, Runx2 and Col10a1 in MSCs induced for chondrogenesis under normoxic (21% O2, the air) and hypoxic (1% O2) conditions by quantitative RT-PCR. MSCs induced under hypoxic conditions exhibited significantly higher mRNA levels of Sox9, Col2a1 and aggrecan compared to those of MSCs under normoxic conditions (p < 0.05) (Figure 1a). By contrast, hypoxic conditions decreased the mRNA levels of Runx2 and Col10a1 compared to normoxic culture (p < 0.01). Moreover, histological sections stained with Alcian blue also demonstrated that hypoxic conditions had increased proteoglycan synthesis compared to normoxic culture on day 7 and 14 of chondrogenesis (Figure 1b). ICC further demonstrated that hypoxic culture of MSCs increased type II collagen expression (Figure 1c), but decreased type X collagen expression compared to normoxic culture (Figure 1d). Together, these data suggested that hypoxic conditions increased chondrogenesis and suppressed expression of markers associated with endochondral ossification.
Hypoxic culture enhanced chondrogenesis and suppressed expression of markers of endochondral ossification in MSCs.
MSCs (aliquots of 2.5 × 105) were pelleted and induced in chondrogenic differentiation medium under normoxic (Nor, 21% O2) and hypoxic (Hyp, 1% O2) conditions. (a) Quantitative RT-PCR for mRNA levels on day 7 of induction. (b) (Left panel) Alcian blue staining on days 7 and 14 of induction. (Right panel) Quantification by Image-Pro Plus. (c) (Left panel) ICC staining for type II collagen on days 7 and 14 of induction. (Right panel) Quantification by Image-Pro Plus. (d) (Left panel) ICC staining for type X collagen on days 7 and 14 of induction. (Right panel) Quantification by Image-Pro Plus. Scale bars = 100 microns. [Values are mean ± SE; *, p < 0.05 and **, p < 0.01 indicate significant variance (Mann Whitney U test) compared to MSCs incubated under Nor conditions] (Three pellets were conducted at different times for each MSC line. Experiments were performed using MSCs from three individuals and representative data from individual #2 are shown).
Hypoxic culture suppressed chondrogenesis-induced apoptosis
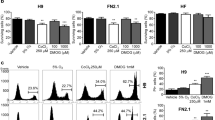
Chondrogenesis has been demonstrated to induce apoptosis, which is associated with the expression of markers of endochondral ossificatio15. To examine whether the decreased expression of endochondral ossification markers was associated with reduced apoptosis during chondrogenesis, MSC pellets were subjected to apoptotic assays. TUNEL assay of microscopic sections revealed that hypoxic conditions significantly reduced apoptosis of MSCs on days 2, 7 and 14 of chondrogenesis compared to that of MSCs under normoxic culture (p < 0.01) (Figure 2a). Annexin-V/PI staining of MSCs recovered from the pellets revealed that the apoptotic rates on day 2 of chondrogenesis were 27.75% and 56.06% for hypoxic and normoxic cultures, respectively. These data suggest that hypoxic culture suppressed chondrogenesis-induced apoptosis in MSCs (Figure 2b).
Hypoxic culture suppressed chondrogenesis-induced apoptosis.
MSCs (aliquots of 2.5 × 105) were pelleted and induced in chondrogenic differentiation medium under normoxic (Nor, 21% O2) and hypoxic (Hyp, 1% O2) conditions. (a) (Left panel) TUNEL assay at indicated time of induction. (Right panel) Quantification by Image-Pro Plus. (b) Pellets from 2 days of normoxic or hypoxic culture were digested with collagenase and single cell suspension prepared. Apoptosis and cell death were evaluated quantitatively by flow cytometry after Annexin-V/PI staining. Data are gated for viable cells (PI- and Annexin V-), apoptotic cells (PI- and Annexin V+) and dead cells (PI+ and Annexin V+ or Annexin V-). Scale bars = 100 microns. [Values are mean ± SE; *, p < 0.05 and **, p < 0.01 indicate significant variance (Mann Whitney U test) compared to MSCs incubated under Nor conditions] (Three pellets were conducted at different times for each MSC line. Experiments were performed using MSCs from three individuals and representative data from individual #2 are shown).
Hypoxic culture activates the PI3K/Akt/FoxO pathway which suppressed caspase-8 and caspase-3 activation
Since the hypoxia-dependent protection from apoptosis correlates with the activation of the PI3K/Akt pathwa12, we therefore examined whether hypoxia activated the PI3K/Akt pathway in MSCs. Western blotting analysis revealed that the levels of phosphorylated Akt were significantly increased between 12 and 72 h of chondrogenesis under hypoxic conditions compared to those under normoxic conditions (Figure 3a). Similarly, the levels of phosphorylated FoxO1/O3 but not GSK-3β, or BAD were also increased under hypoxic culture compared to those under normoxic culture. Addition of wortmannin, a PI3K inhibitor, suppressed the hypoxia-induced phosphorylation of Akt and FoxO1/O3 (Figure 3b and data not shown).
Hypoxic culture activates PI3K/Akt/FoxO pathway associated with inactivation of caspases.
MSCs (2.5 × 105) were pelleted and induced in chondrogenic differentiation medium under normoxic (Nor, 21% O2) and hypoxic (Hyp, 1% O2) conditions. (a) and (b) MSC pellets from indicated time of normoxic or hypoxic culture without or with wortmanin (WMN) treatment were digested with collagenase followed by protein lysis. Protein lysates (20 ug) were analyzed for levels of phosphorylated Akt (pAkt) and phosphorylated FoxO1/O3 (pFoxO1/O3) by Western blotting. Blots were cropped in the figure, but the gels were run under the same experimental conditions and full-length blots are presented in Supplementary data. (c) (Left panel) ICC staining of activated caspase-8 on day 2 of induction. (Right panel) Quantification by Image-Pro Plus. Scale bars = 100 microns. (d) (Left panel) ICC staining of activated caspase-3 on day 2 of induction. (Right panel) Quantification by Image-Pro Plus. Scale bars = 100 microns. [Values are mean ± SE; *, p < 0.05 and **, p < 0.01 indicate significant variance (Kruskal Wallis H test) compared to MSCs incubated under Nor conditions] (Three pellets were conducted at different times for each MSC line. Experiments were performed using MSCs from three individuals and representative data from individual #2 are shown).
Since caspases are activated during chondrogenesis-induced apoptosis in MSCs13,16,17, we examined whether hypoxic culture reduced the activation of caspases. MSCs in the 2-day chondrogenic pellets under hypoxic conditions showed lower activated caspase-8 and caspase-3 but not caspase-9 than those under normoxic conditions (Figure 3c and 3d, data not shown). As expected, treatment of hypoxic MSCs with wortmannin increased the expression of active caspase-8 and caspase-3 in the 2-day chondrogenic pellets. These data suggested that hypoxic conditions activated the PI3K/Akt pathway which inhibited activation of caspase-8 and caspase-3 during chondrogenesis of MSCs. To clarify whether PI3K/Akt activation and the phosphorylation of FoxO (inactivation) were involved in hypoxia-mediated suppression of apoptosis, MSCs under hypoxic culture were treated with PI3K inhibitor (wortmannin), vehicle, or either of the FoxO transportation inactivators (trans-4-hydroxy-2-nonenal (4-HNE), Leptomycin B (LMB)) for 2 h and subsequently analyzed with TUNEL assay. Inhibition of PI3K abrogated hypoxic culture-induced phosphorylation of Akt and downstream FoxO (Figure 3b) and augmented apoptosis during chondrogenesis (Fig. 4b). Similar to PI3K inhibition, blockage of FoxO inactivation with 4-HNE or LMB increased apoptosis of MSCs under hypoxic conditions (Figure 4b). Inhibition of PI3K or blockage of FoxO inactivation also repressed hypoxic culture-induced inactivation of caspase-8 (Figure 4c) and caspase-3 (Figure 4d). However, treatment with inhibitors of PI3K/Akt pathway or FoxO inactivation in normoxic culture did not induce changes in apoptosis and activation of caspase-8 and caspase-3 (Figure 4b–4d). The results suggested that hypoxic conditions suppressed activation of caspases through activation of the PI3K/Akt/FoxO pathway.
Hypoxic culture suppressed apoptosis and activation of caspases via activation of the PI3K/Akt/pFoxO pathway.
MSCs (2.5 × 105) were pelleted and induced in chondrogenic differentiation medium under normoxic (Nor, 21% O2) and hypoxic (Hyp, 1% O2) conditions in the absence or presence of wortmannin (WMN), 4-Hydroxynonenal(4-HNE) or Leptomycin B (LMB). (a) Quantitative RT-PCR for mRNA levels on days 2 and 7 of induction. (b) (Left panel) Analysis for apoptosis with TUNEL assay on day 2 of induction. (Right panel) Quantification by Image-Pro Plus. (c) (Left panel) ICC staining for active caspase-8 on day 2 of induction. (Right panel) Quantification by Image-Pro Plus. (d) (Left panel) ICC staining for active caspase-3 on day 2 of induction. (Right panel) Quantification by Image-Pro Plus. Scale bars = 100 microns. [Values are mean ± SE; *, p < 0.05 and **, p < 0.01 indicate significant variance (Kruskal Wallis H test) compared to MSCs incubated under Nor conditions] (Three pellets were conducted at different times for each MSC line. Experiments were performed using MSCs from three individuals and representative data from individual #2 are shown).
Hypoxic culture enhanced chondrogenesis and suppressed expression of markers associated with endochondral ossification through activation of the PI3K/Akt/FoxO pathway
To clarify whether hypoxic culture activates the PI3K/Akt/FoxO pathway, enhances chondrogenesis and suppresses expression of markers of endochondral ossification during chondrogenesis, MSCs under hypoxic conditions were treated with wortmannin, 4-HNE, LMB, or vehicle and analyzed for chondrogenic potential and expression of markers associated with endochondral ossification. Interestingly, inhibition of PI3K/Akt pathway or FoxO inactivation in MSCs under hypoxic conditions reduced expression of Sox9, Col2a1 and aggrecan; but increased expression of Col10a1 and Runx2 (Figure 4a). Similarly, histological sections of MSCs incubated with inhibitors of PI3K/Akt pathway or FoxO inactivation in hypoxic culture showed a lower intensity of Alcian blue staining and ICC of type II collagen immunostaining than those treated with vehicle under hypoxic conditions (Figure 5a, 5b). They also showed higher expression of type X collagen than the vehicle control (Figure 5c). However, treatment with inhibitors of PI3K/Akt pathway or FoxO inactivation in normoxic culture did not induce changes in the expression of Sox9, Col2a1, aggrecan; Col10a1 and Runx2, Alcian blue staining and ICC of type II and X collagen (Figure 4a, 5a–5c).
Hypoxic culture enhanced chondrogenesis and suppressed expression of markers associated with endochondral ossification through activation of the PI3K/Akt/FoxO pathway.
MSCs (2.5 × 105) were pelleted and induced in chondrogenic differentiation medium for 7 days under normoxic (Nor, 21% O2) and hypoxic (Hyp, 1% O2) conditions in the absence or presence of wortmannin (WMN), 4-Hydroxynonenal (4-HNE) or Leptomycin B (LMB). (a) (Left panel) Alcian blue staining. (Right panel) Quantification by Image-Pro Plus. (b) (Left panel) ICC staining for type II collagen. (Right panel) Quantification by Image-Pro Plus. (c) ICC staining for type X collagen. (Right panel) Quantification by Image-Pro Plus. Scale bars = 100 microns. [Values are mean ± SE; *, p < 0.05 and **, p < 0.01 indicate significant variance (Kruskal Wallis H test) compared to MSCs incubated under Nor conditions] (Three pellets were conducted at different times for each MSC line. Experiments were performed using MSCs from three individuals and representative data from individual #2 are shown).
To further assess the role of Akt, we also demonstrated that knockdown of Akt by lentiviral transduction of MSCs under hypoxic conditions reduced Akt expression and FoxO phosphorylation (Figure 6a), increased apoptosis (Figure 6c), augmented activated caspase-8 and capase-3 levels (Figure 6d, 6e), decreased expression of Sox9, Col2a1, aggrecan, but increased expression of Col10a1 and Runx2 (Figure 6b), reduced Alcian blue staining (Figure 7a) and ICC of type II collagen (Figure 7b) and increased ICC of type X collagen (Figure 7c). Taken together, these data suggested that hypoxic culture enhanced MSC chondrogenesis and suppressed expression of markers associated with endochondral ossification through activation of the PI3K/Akt/FoxO pathway.
Knockdown of Akt by lentiviral transduction in hypoxic culture induced a decrease in phosphorylation of FoxO.
MSCs (2.5 × 105) transduced with control scrambled shRNA (Scr) or Akt-specific shRNAs, siAkt(1) and siAkt(2), were pelleted and induced in chondrogenic differentiation medium under normoxic (Nor, 21% O2) and hypoxic (Hyp, 1% O2) conditions. (a) Cell pellets of 2 days were digested with collagenase followed by protein lysis. Protein lysate was assayed with western blotting analysis. Blots were cropped in the figure, but the gels were run under the same experimental conditions and full-length blots are presented in Supplementary data. (b) Cell pellets were digested with collagenase followed by extraction of mRNA and quantitative RT-PCR for mRNA levels. (c) (Left panel) Cell pellets of 7 days were assayed for apoptosis by TUNEL assay. (Right panel) Quantification by Image-Pro Plus. (d) (Left panel) Cell pellets of 7 days were assayed by ICC staining for active caspase-8. (Right panel) Quantification by Image-Pro Plus. (e) (Left panel) Cell pellets of 7 days were assayed for active caspase-3 by ICC staining. (Right panel) Quantification by Image-Pro Plus. Scale bars = 100 microns. [Values are mean ± SE; *, p < 0.05 and **, p < 0.01 indicate significant variance (Kruskal Wallis H test) compared to MSCs incubated under Nor conditions] (Three pellets were conducted at different times for each MSC line. Experiments were performed using MSCs from three individuals and representative data from individual #2 are shown).
Knockdown of Akt by lentiviral transduction in hypoxic culture reduced chondrogenesis of MSCs.
MSCs (2.5 × 105) transduced with control scrambled shRNA (Scr) or Akt-specific shRNAs, siAkt(1) and siAkt(2), were pelleted and induced in chondrogenic differentiation medium under normoxic (Nor, 21% O2) and hypoxic (Hyp, 1% O2) conditions for 7 days. (a) (Left panel) Cell pellets were stained with Alcian blue. (Right panel) Quantification by Image-Pro Plus. (b) (Left panel) Cell pellets were stained by ICC staining for type II collagen. (Right panel) Quantification by Image-Pro Plus. (c) (Left panel) Cell pellets were stained by ICC staining for type X collagen. (Right panel) Quantification by Image-Pro Plus. Scale bars = 100 microns. [Values are mean ± SE; *, p < 0.05 and **, p < 0.01 indicate significant variance (Kruskal Wallis H test) compared to MSCs incubated under Nor conditions] (Three pellets were conducted at different times for each MSC line. Experiments were performed using MSCs from three individuals and representative data from individual #2 are shown).
Discussion
Whereas effects of hypoxia on MSC chondrogenesis have been investigated previously18, most of the effects and the detailed mechanisms remain unknown. In the current study, we demonstrated that hypoxic conditions compared to normoxic conditions enhanced MSC chondrogenesis with increased expression of chondrogenic genes and proteins. Furthermore, hypoxic conditions inhibited endochondral ossification with reduced expression of associated genes and proteins. We also demonstrated that hypoxia mediated MSC differentiation by reducing apoptosis via activating the PI3K/Akt/FoxO pathway. Previously, we had demonstrated that MSCs underwent apoptosis upon induction for chondrogenic differentiation13. Apoptosis has also been demonstrated as a general phenomenon that occurs during endochondral differentiation of chondrocytes and a possible mechanism has been described partially in several previous studies19. One study demonstrated that differentiation of chondrocytes involved higher Fas receptor and caspase protein expression which are two markers of apoptosis. Induced apoptosis in chondrocytes progression to endochondral ossification may involve Fas and caspase pathway mediated signalling20. Other studies showed that both the Wnt/beta-catenin and Indian hedgehog (Ihh) signalling pathways play essential roles in the process of endochondral ossification. Beta-catenin is required upstream of Ihh signalling for chondrocyte survival and inhibition of apoptosis21. Consistent with and expanding previous results, we first found that MSCs underwent apoptosis via activating caspase-8 and caspase-3 during chondrogenesis. Moreover, hypoxic conditions inhibited chondrogenesis-induced apoptosis, dependent on the activation of the PI3K/Akt/FoxO pathway. Therefore, these data may help to develop strategies in controlling chondrogenesis and endochondral ossification.
Articular hyaline cartilage is avascular in nature, having no nerves or blood supply; therefore ooxygen could only diffuse from the synovial fluid. Thus, there appears to be a gradient of decreasing oxygen tension from the surface of the articular cartilage to the subchondral bone and the physiological oxygen tension of articular chondrocytes is 5–10% at the surface and possibly < 1% in the deepest layer22. The chondrocytes of articular cartilage can survive with maintained phenotype under low oxygen tension23. Moreover, increase of oxygen tension, which occurs in osteoarthritis after invasion of neovessels into tidemarks, often causes osteochondral angiogenesis within subchondral spaces24. These processes may induce chondrocyte apoptosis followed by an increase in bone density of subchondral bone and spur formation24,25. This process was similar to the process of endochondral ossification observed in growth plate, where the differentiated chondrocytes may undergo apoptosis while proceeding into endochondral ossification19,26. Taken together, these data, consistent with previous reports27, suggest that MSCs, chondrogenic progenitor cells, or chondrocytes can survive under hypoxic conditions and undergo apoptosis with associated endochondral ossification under normoxic conditions utilizing mechanisms elucidated in these studies.
In the current study, we first demonstrated that the hypoxic condition could inhibit apoptosis which enhanced chondrogenic differentiation and reduced endochondral ossification. We have also uncovered significant differences in the activation of caspase-8 and caspase-3 between hypoxic and normoxic cultures. The data demonstrated lower levels of caspase-8 and caspase-3 during chondrogenesis under hypoxic conditions. We further demonstrated a greater level of Akt and FoxO phosphorylation in MSCs under hypoxic conditions compared to those under normoxic conditions. Moreover, co-treatment of hypoxic culture with the potent PI3K inhibitor, wortmannin, or knockdown of Akt completely blocked the effect of hypoxic culture on preventing chondrogenesis-induced apoptosis in differentiated MSCs. Furthermore, MSCs treated with either of two blockers of FoxO inactivation under hypoxic conditions also increased apoptosis, reversed the expression of chondrogenic genes and increased the expression of genes associated with endochondral ossification. We have also examined the phosphorylated levels of many downstream signalling molecules of PI3K/Akt such as GSK-3β, Bad and activated caspase-9; however, no significant differences were detected (data not shown). Taken together, these data suggest that hypoxic conditions inhibit chondrogenesis-induced apoptosis via the specific activation of the PI3K/Akt/FoxO pathway.
Although the PI3K/Akt pathway is considered as a therapeutic target in autoimmune disease such as rheumatoid arthritis28,29, its roles in maintaining chondrocyte phenotype or inhibiting osteoarthritic change remain to be clarified. The current study demonstrated that hypoxic conditions activate PI3K/Akt/FoxO pathway which inhibits chondrogenesis-induced apoptosis. Hypoxia-activated PI3K/Akt/FoxO pathway also promotes expression of chondrogenic genes and proteins, but suppresses genes associated with endochondral ossifications. Similar to the current study, the expression of Sox9, col2a1 and aggrecan in prechondrogenic cells30 and chondrocytes14 is inhibited by blocking PI3K/Akt pathway and enhanced by PI3K/Akt activation. It has also been demonstrated that PI3K/Akt regulated col2a1 and aggrecan by modulating Sox9 expression and transcriptional activity in nucleus pulposus cells31. Thus, reagents that modualte PI3K/Akt pathway may be applied to control chondrogenesis and enhance cartilage regeneration. Since chondrocyte apoptosis and endochondral ossification have been implicated in the aetiology of osteoarthritis32, the current data suggest that PI3K/Akt/FoxO activated by hypoxic culture may also have beneficial effects on articular cartilage. In healthy articular cartilage, chondrocytes resist terminal differentiation. By contrast, chondrocytes in osteoarthritic cartilage progressively develop hypertrophy and angiogenesis and focal calcification of joint cartilage are initiated33. Increased expression of the Akt inhibitor tribbles homolog, TRB3, in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis34. Moreover, activation of the PI3K/Akt pathway by Wnt signalling has also been shown to be involved in endochondral ossification process by inhibiting apoptosis in C2C12 uncommitted bipotential cells or MC3T3-E1 preosteoblast cells35. These data suggest that the PI3K/Akt pathway plays a key role in modulating chondrocyte phenotype or endochondral ossification and is thus a therapeutic target for disease-modifying osteoarthritis therapies.
Hypoxia-induced up-regulation of chondrogenic genes such as Sox9, Col2a1 and aggrecan are seemed to be almost completely abolished by Akt knockdown. Notably, Runx2 and Col10a1 gene expression are still down-regulated by hypoxia even with Akt knockdown, suggesting that there is another very important pathway or factor to suppress hypertrophy of chondrocyte in hypoxia. One of the alternative pathways involved in suppressing hypertrophy of chondrocyte is the HIF-1α-Twist pathway. Our previous data demonstrated that Twist up-regulated by HIF-1α inhibits transcription of Runx2 and its downstream targets both in vitro36 and in vivo37. These data together suggest hypoxia controls lineage differentiation of stem cells through HIF-1α-dependent and-independent pathways.
4-HNE has been hypothesized by several studies to play a key role in signal transduction of FoxO in cells38,39. It can trigger cell death via induction of caspase enzymes, leading to cell apoptosis and necrosis. Export of some nuclear proteins such as FoxO, which contain nuclear export sequence (NES), requires the binding of an exportin (CRM1) and the RanGTP to form the heterotrimeric complex. After GTP hydrolysis and the release of the NES-protein, the NES-containing proteins diffuse into the cytoplasm and start to function, while the CRM1-RanGDP complex diffuses back into the nucleus40. CRM1-mediated protein export can be inhibited by LMB. Thus, we applied this compound, 4-HNE, to block the nuclear export mechanism and thereby interrupt the transport of FoxO to the cytoplasm41,42. The effects of 4-HNE and LMB-induced activation of FoxO on MSC chondrogenseis was similar to the effects of PI3K inhibitor, wortmannin. Therefore, these data suggest that FoxO plays an important role in mediating the effects of the activation of PI3K/Akt by hypoxic culture conditions on MSC chondrogenesis.
Previously, Akt activation was reported to induce the expression of HIF-1 or HIF-2 through Akt/PI3K/HIF-1 or Akt/Bcl-xL/HIF-2 pathway11,43. According to the previous studies about the mechanism of apoptosis, the upstream signal PI3K/Akt may affect the HIF-1 or HIF-2 expression during the chondrogenic differentiation under hypoxic conditions11,43,44,45. We also examined HIF signals, but the results demonstrated no significant differentiation (data not shown). The current study is not focused on investigating the discrepancy between our study and others. However, it seems that hypoxic conditions may have a protective effect during chondrogenic differentiation.
In conclusion, MSCs-induced chondrogenic differentiation can be enhanced under hypoxic conditions. We have demonstrated the enhancing effect of chondrogenic differentiation under the environment of 1% O2. The PI3K/Akt/FoxO signalling pathway may play a crucial role in the chondrogenic differentiation of MSCs and the PI3K/Akt/FoxO dependent anti-apoptotic effect can enhance more chondrogenesis under hypoxic conditions. These studies may provide new strategies in tissue engineering for therapeutics of cartilage injury and degeneration.
Methods
Culture of human bone marrow-derived mesenchymal stem cells
These studies were approved by the Institutional Review Board of Taipei Veterans General Hospital, with informed consent obtained from donors who provided bone marrow aspirates. The donors are three male Asians with age ranged from the third to fifth decade, who received a spine surgery for spinal disorders in Taipei Veterans General Hospital. Primary MSCs from bone marrow were prepared as described previously6. Cells were seeded at 50 cells per cm2 and grown in complete culture medium [CCM: α-MEM (α-minimal essential medium; Gibco-BRL, Gaithersburg, MD), supplemented with 16.6% fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine] with medium change twice per week. Hypoxic gas mixture was maintained in an incubator with two air sensors, one for CO2 and the other for O2. The O2 concentration was achieved and maintained using delivery of nitrogen gas (N2) generated from a tank containing pure N2. When O2 percentage rose above the desired set level, N2 gas was automatically injected into the system to displace the excess O2.
Chondrogenic differentiation and histogram-based quantification
For in vitro differentiation into chondrocytes, equal aliquots of MSCs (2.5 × 105 cells) in 2 mL CCM were concentrated at 500 g for 10 min and the pelleted cells were incubated at 37°C, 5% CO2. After overnight incubation, the cells had formed an essentially spherical aggregate that did not adhere to the walls of the tube. The cells were induced with chondrogenic induction medium [CIM, cell pellets in serum-free α-MEM supplemented with ITS+ (GIBCO), 10 ng/mL TGF-β1 (Preprotech, Rocky Hill, NJ), 10−7 M dexamethasone (Sigma) and 50 μg/mL ascobate-2-phosphate (Sigma)]13. The protocol for the chondrogenic differentiation was conducted using the MSCs from three individuals for each time and repeated for at least three or four different times. Cell pellets induced with CIM were prepared for paraffin sections and used for Alcian blue staining and immunocytochemistry (ICC). For ICC, the paraffin sections were initially incubated with blocking serum, probed with monoclonal antibodies against human type II collagen, type X collagen, active caspase-8 and active caspase-3 (Chemicon; CA), then reacted with an alkaline phosphatase (AP)-conjugated goat anti-mouse IgG antibody and finally processed for AP-Vector Red staining (Vector; CA). The images were saved and analyzed with Image-Pro Plus 4.5 software (Media Cybernetics; Silver Spring, MD) using histogram-based quantification7.
Reverse transcription and real-time PCR
Real-time RT-PCR was performed as described27. Briefly, total RNA was extracted using TRIzol kit (Invitrogen, CA). RNA was reverse transcribed in a final volume of 20 μL using 0.5 μg of oligo dT and 200 U Superscript III RT (Invitrogen) for 30 min at 50°C, followed by 2 min at 94°C to inactivate the reverse transcriptase. Real-time PCR amplification was carried out in a total volume of 25 μL containing 0.5 μM of each primer, 4 mM MgCl2, 12.5 μL of LightCycler™ FastStart DNA Master SYBR green I (Roche Molecular Systems, Alameda, CA) and 10 μL of 1:20 diluted cDNA. PCR reactions were prepared in duplicate and heated to 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 1 min and extension at 72°C for 20 sec. Standard curves (cycle threshold values versus template concentration) were prepared for each target gene and for the endogenous reference (GAPDH) in each sample. Quantification of the unknown samples was performed using the LightCycler Relative Quantification Software version 3.3 (Roche). Primer sequences are listed in Table I.
Western blotting
Cell extracts were prepared with M-PER (Pierce, IL) plus protease inhibitor cocktail (Halt™; Pierce) and protein concentrations were determined using the BCA assay (Pierce). Protein lysates (20 μg) were separated on SDS–10% polyacrylamide gels, transferred onto PVDF membranes, blocked with 5% blotting grade milk (Bio-Rad, Hercules, CA) in TBST (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, 1% Tween 20), probed with the indicated primary antibodies, reacted with corresponding secondary antibodies and detected by using a chemiluminescence assay (Millipore). Membranes were exposed to X-ray film (Amersham Pharmacia Biotech, NJ) for visualization.
TUNEL assay
Cell pellets were harvested, washed twice with PBS, fixed in 4% paraformaldehyde for 3 h at room temperature, paraffin embedded and sectioned. Sections (4 μm-thick) were analyzed with TUNEL assay (In Situ Cell Death Detection Kit, Roche) to detect apoptotic cells with high concentrations of DNA 3′-OH ends.
Trypan blue staining and Annexin-V/PI flow cytometry
Trypan blue exclusion method was used to determine the total viable cells in each pellet and the ratio of viability. Flow cytometric analysis of Annexin-V and propidium iodide (PI) staining which detects the exposure of phosphatidylserine and the exposed DNA content in apoptotic cells, respectively was performed using an Annexin-V-fluos kit (Roche, Indianapolis, IN).
Lentiviral-Mediated RNAi
Expression plasmids and the bacteria clone for Akt shRNA (TRCN0000010162 and TRCN0000010174) were provided by the National Science Council in Taiwan. Lentiviral production was done by transfection of 293 T cells using Lipofectamine 2000 (LF2000; Invitrogen, Carlsbad, CA). Supernatants were collected 48 h after transfection and filtered. Subconfluent cells were infected with lentivirus in the presence of 8 μg/mL polybrene (Sigma-Aldrich). At 24 h post-infection, we removed medium and replaced with fresh growth medium containing puromycin (1 μg/mL) and selected for infected cells for 48 h.
Statistical analysis
Most of the experiments including real-time PCR, western blotting, TUNEL assay, flow cytometry and other staining methods were performed by one observer with standardized protocols. All experiments were performed with three or more different times for each cell line to reduce the chance variance. Since phenotypes or differentiation potentials varied among the MSCs collected from different individuals46,47, the statistical analyses were conducted among different pellets from the same MSC line but cultured at different times rather than pellets derived from different MSC lines. The quantitative results were represented as the mean ± the standard error (SE) of at least three experiments at different times. Comparisons were made using Mann Whitney U test between 2 groups. Other comparisons among more than 2 groups were made using Kruskal Wallis H test. P < 0.05 is considered significant.
Role of funding source
This research was funded by grants from Veterans General Hospital-Taipei (V99E1-011); National Science Council (98-3111-B-010-001) and National Yang-Ming University, Ministry of Education. This work is assisted in part by the Division of Experimental Surgery of the Department of Surgery, Taipei Veterans General Hospital. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
References
Najafipour, H. & Ferrell, W. R. Comparison of synovial PO2 and sympathetic vasoconstrictor responses in normal and acutely inflamed rabbit knee joints. Exp. Physiol 80, 209–220 (1995).
Kiaer, T., Gronlund, J. & Sorensen, K. H. Subchondral pO2, pCO2, pressure, pH and lactate in human osteoarthritis of the hip. Clin. Orthop. Relat Res. 149–155 (1988).
Wakitani, S. et al. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng 4, 429–444 (1998).
Nejadnik, H., Hui, J. H., Feng Choong, E. P., Tai, B. C. & Lee, E. H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am. J. Sports Med. 38, 1110–1116 (2010).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Sekiya, I. et al. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 20, 530–541 (2002).
Johansson, A. C., Visse, E., Widegren, B., Sjogren, H. O. & Siesjo, P. Computerized image analysis as a tool to quantify infiltrating leukocytes: a comparison between high- and low-magnification images. J. Histochem. Cytochem. 49, 1073–1079 (2001).
Chiou, S. H. et al. Identification of CD133-positive radioresistant cells in atypical teratoid/rhabdoid tumor. PLoS. One. 3, e2090 (2008).
Davidson, D. et al. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J. Biol. Chem. 280, 20509–20515 (2005).
Robins, J. C. et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 37, 313–322 (2005).
Kanichai, M., Ferguson, D., Prendergast, P. J. & Campbell, V. A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J. Cell Physiol 216, 708–715 (2008).
varez-Tejado, M. et al. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J. Biol. Chem. 276, 22368–22374 (2001).
Wang, C. Y. et al. Apoptosis in chondrogenesis of human mesenchymal stem cells: effect of serum and medium supplements. Apoptosis. 15, 439–449 (2010).
Kita, K., Kimura, T., Nakamura, N., Yoshikawa, H. & Nakano, T. PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes Cells 13, 839–850 (2008).
Gibson, G. Active role of chondrocyte apoptosis in endochondral ossification. Microsc. Res. Tech. 43, 191–204 (1998).
Pelletier, J. P. et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 43, 1290–1299 (2000).
Sharif, M., Whitehouse, A., Sharman, P., Perry, M. & Adams, M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 50, 507–515 (2004).
Meyer, E. G., Buckley, C. T., Thorpe, S. D. & Kelly, D. J. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J. Biomech. 43, 2516–2523 (2010).
Cheung, J. O. et al. Apoptosis of terminal hypertrophic chondrocytes in an in vitro model of endochondral ossification. J. Pathol. 201, 496–503 (2003).
Aizawa, T., Kon, T., Einhorn, T. A. & Gerstenfeld, L. C. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J. Orthop. Res. 19, 785–796 (2001).
Mak, K. K., Chen, M. H., Day, T. F., Chuang, P. T. & Yang, Y. Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development 133, 3695–3707 (2006).
Silver, I. A. Measurement of pH and ionic composition of pericellular sites. Philos. Trans. R. Soc. Lond B Biol. Sci. 271, 261–272 (1975).
Lafont, J. E. Lack of oxygen in articular cartilage: consequences for chondrocyte biology. Int. J. Exp. Pathol. 91, 99–106 (2010).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology. (Oxford) 49, 1852–1861 (2010).
Suri, S. et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 66, 1423–1428 (2007).
Kouri, J. B., Aguilera, J. M., Reyes, J., Lozoya, K. A. & Gonzalez, S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. J. Rheumatol. 27, 1005–1019 (2000).
Tsai, C. C. et al. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 117, 459–469 (2011).
Tas, S. W., Remans, P. H., Reedquist, K. A. & Tak, P. P. Signal transduction pathways and transcription factors as therapeutic targets in inflammatory disease: towards innovative antirheumatic therapy. Curr. Pharm. Des 11, 581–611 (2005).
Wu, T. & Mohan, C. The AKT axis as a therapeutic target in autoimmune diseases. Endocr. Metab Immune. Disord. Drug Targets. 9, 145–150 (2009).
Fujita, T., Fukuyama, R., Enomoto, H. & Komori, T. Dexamethasone inhibits insulin-induced chondrogenesis of ATDC5 cells by preventing PI3K-Akt signaling and DNA binding of Runx2. J. Cell Biochem. 93, 374–383 (2004).
Cheng, C. C. et al. PI3K/AKT regulates aggrecan gene expression by modulating Sox9 expression and activity in nucleus pulposus cells of the intervertebral disc. J. Cell Physiol 221, 668–676 (2009).
Kawaguchi, H. Regulation of osteoarthritis development by Wnt-beta-catenin signaling through the endochondral ossification process. J. Bone Miner. Res. 24, 8–11 (2009).
Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 12, 216 (2010).
Li, X. et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 60, 513–523 (2009).
Almeida, M., Han, L., Bellido, T., Manolagas, S. C. & Kousteni, S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 280, 41342–41351 (2005).
Yang, D. C. et al. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS. One. 6, e23965 (2011).
Yang, D. C. et al. Twist controls skeletal development and dorsoventral patterning by regulating runx2 in zebrafish. PLoS. One. 6, e27324 (2011).
Nakashima, I. et al. 4-hydroxynonenal triggers multistep signal transduction cascades for suppression of cellular functions. Mol. Aspects Med. 24, 231–238 (2003).
Vaillancourt, F. et al. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res. Ther. 10, R107 (2008).
Yoneda, Y., Hieda, M., Nagoshi, E. & Miyamoto, Y. Nucleocytoplasmic protein transport and recycling of Ran. Cell Struct. Funct. 24, 425–433 (1999).
Tanno, M., Sakamoto, J., Miura, T., Shimamoto, K. & Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 282, 6823–6832 (2007).
Zanella, F. et al. An HTS approach to screen for antagonists of the nuclear export machinery using high content cell-based assays. Assay. Drug Dev. Technol. 5, 333–341 (2007).
Bohensky, J. et al. Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 60, 1406–1415 (2009).
Malladi, P., Xu, Y., Chiou, M., Giaccia, A. J. & Longaker, M. T. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng 13, 1159–1171 (2007).
Khan, W. S., Adesida, A. B. & Hardingham, T. E. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res. Ther. 9, R55 (2007).
Siddappa, R., Licht, R., van, B. C. & de, B. J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 25, 1029–1041 (2007).
Jaager, K., Islam, S., Zajac, P., Linnarsson, S. & Neuman, T. RNA-seq analysis reveals different dynamics of differentiation of human dermis- and adipose-derived stromal stem cells. PLoS. One. 7, e38833 (2012).
Author information
Authors and Affiliations
Contributions
H.H.L., T.H.Y. and S.C.H. designed the studies. H.H.L., C.C.C. and M.J.S. collected and assembled the data. H.H.L., C.C.C., M.J.S., J.P.W., Y.T.C., T.H.Y. and S.C.H. analysed the data and interpreted findings. H.H.L., T.H.Y. and S.C.H. wrote the manuscript. H.H.L., C.C.C., M.J.S., J.P.W., Y.T.C., T.H.Y. and S.C.H. reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lee, HH., Chang, CC., Shieh, MJ. et al. Hypoxia Enhances Chondrogenesis and Prevents Terminal Differentiation through PI3K/Akt/FoxO Dependent Anti-Apoptotic Effect. Sci Rep 3, 2683 (2013). https://doi.org/10.1038/srep02683
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02683
This article is cited by
-
Regenerative capacity of trophoblast stem cell-derived extracellular vesicles on mesenchymal stem cells
Biomaterials Research (2023)
-
Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies
Stem Cell Research & Therapy (2023)
-
Chondrogenic primed extracellular vesicles activate miR-455/SOX11/FOXO axis for cartilage regeneration and osteoarthritis treatment
npj Regenerative Medicine (2022)
-
In silico effect of Korean medicinal phytocompounds on gene targets of osteoarthritis
Advances in Traditional Medicine (2022)
-
A scaffold-free approach to cartilage tissue generation using human embryonic stem cells
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.