Abstract
In the study, we analyzed role of p53 in predicting outcome in visceral metastasis breast cancer (VMBC) patients. 97 consecutive VMBC patients were studied. P53 positivity rate was 29.9%. In the p53-negative group, median disease free survival (DFS) and time from primary breast cancer diagnosis to death (OS1), time from metastases to death (OS2) were 25, 42.5 and 13.5 months, respectively. In the p53-positive group, they were 10, 22 and 8 months, respectively. Statistically significant differences in DFS and OS1 were detected between the p53-negative and p53-positive subtypes. However, p53 appears to have no influence on OS2. In Cox regression analysis, p53 expression and TNM stage were predictive factors of DFS. In the multivariate analysis, p53 expression and the duration of DFS correlated with OS1, but not for OS2. Taken together, our data indicate p53 showing predicting role in OS1 for VMBC, but not for OS2.
Similar content being viewed by others
Introduction
Although significant advances in survival have been achieved with adjuvant treatments in patients with early-stage primary breast carcinoma, patients with recurrent metastatic breast carcinoma usually die of their disease. Several factors, such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), age, stage, grade, time since metastatic development and the site of metastasis, have all been well identified as predictive criteria for the prognosis1. P53 is also well studied marker in breast cancer. But its significance in predicting clinical outcome remains controversial.
The p53 gene is located on the short arm of chromosome 17 and encodes a 375 amino acid nuclear phosphoprotein that prevents propagation of genetically altered cells2. Wild type p53 is a tumor suppressor protein that plays a vital role in regulating genomic stability by controlling the cell cycle and inducing apoptosis when cell damage is beyond repair3,4,5. In normal cells, p53 is expressed in minutes and has a very short half-life by virtue of ubiquitylation and proteasome degradation mediated by MDM26,7. However, missense mutations within the p53 gene result in protein that is stabilized through posttranscriptional modification and accumulation within the cell nucleus. Mutations in the tumor suppressor gene p53 are present in 18%–25% of primary breast carcinomas8. Among the prognostic factors analyzed in studies focusing on breast cancer, an absence of p53 mutations appears to predict longer disease-free (DFS) and overall survival (OS) following primary therapy. With that said, the clinical course of visceral metastatic breast cancer (VMBC) has not been thoroughly investigated and it is unknown whether p53 has significance in VMBC. Knowledge about p53 is important because breast carcinoma is a clinically diverse and heterogeneous disease and the clinical course of these patients varies greatly. Some patients die a short time after they develop recurrent disease, whereas others may live for many years2.
This study attempts to improve the prognostic markers in metastatic VMBC. Unlike ER, PR and HER2, the prognostic and predictive value of p53 in breast cancer, especially in visceral breast cancer, is still under discussion. Some studies have indicated that abnormal p53 immunohistochemical expression, or p53-positive status, was associated with more aggressive tumor features, a higher tumor grade, negative estrogen and progesterone receptor (ER/PR) status and the more aggressive basal subtype9. In contrast, other authors have reported that a p53 mutation does not impact the outcome of early breast cancer and that the evidence is not strong enough for p53 status to be recommended as a routine marker in clinical practice10.
The aim of the present study is (1) to evaluate and compare relationships between p53 status and other tumor characteristics among breast cancer with visceral metastasis, (2) to determine the prognostic value of p53 status within both subgroups and (3) to compare between groups the value of p53 as a prognostic marker in relation to other commonly used prognostic factors.
Results
Patient characterization
The patients' characteristics are summarized in Table 1. The median age was 46 years with a range of 23 to 89. In the group of 97 patients, their age, family history, menstrual status, histological type, TNM stage, number of metastases and status of p53, ER, PR and HER2 were revealed. Among 97 patients, 6 (6.20%) had a family history, 60 (71.89%) had TNM stage III, 60 (71.8%) had multi-organ involvement, 29 (29.9%) were p53 positive, 58 (70.1%) were p53 negative, 65 (67.01%) were ER positive, 53 (54.64%) were PR positive and 30 (30.92%) were HER2 positive.
Correlation of p53 status with tumor stage
The expression of p53 was primarily detected in the nuclear of tumor cells using a semi-quantitative scoring system as previously described11 (Figure 1). The correlations of p53 expression with tumor characteristics are shown in Table 2. High p53 expression was associated with advanced TNM stage (p = 0.011), multiple organ involvement (p = 0.066) and shorter DFS (p = 0.004). However, the expression of p53 was not associated with age, premenopausal status, family history, histological type, or ER, PR and HER2 expression in VMBC patients.
Immunostaining for p53 expression in breast cancer with visceral metastasis.
A: Negative expression, B: Positive expression, Original magnifications: × 400, Scale bars: 20 μm. Wild-type p53 protein is rapidly degraded, but mutated p53 is stable and can be detected with IHC. In Fig. 1B, p53 can be seen in the cells with the brown stain.
Correlation of p53 status with disease free survival
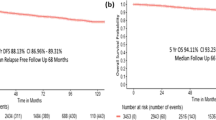
The median follow-up was 57 months after primary treatment. The median time of DFS was 23 months. The median DFS was 10 months for p53-positive patients and 25 months for p53-negative patients (p = 0.004, Figure 2). In univariate analysis, p53 expression and an advanced stage were predictive of a shorter disease free survival (p = 0.006 and 0.008, respectively; Table 3). In the multivariate analysis, p53 expression remained a significant predictor of poor DFS (p = 0.025).
Correlation of p53 status with overall survival
At the end of this study, 26 patients (26.8%) were alive. The median OS1 was 38 months. The median OS1 was 22 months for patients with p53-positive tumors and 42.5 months for patients with p53-negative tumors (p = 0.021, Figure 3). In the univariate analysis, p53 expression, the numbers of metastases and the duration of DFS were predictive of poor survival (p = 0.32; p = 0.027; p = 0.000, respectively; Table 4). In the multivariate analysis, p53 expression and the duration of DFS remained to be the significant predictors of poor OS1 (p = 0.025 and p = 0.031, respectively).
Since p53 expression was strongly associated with DFS and OS1, we also performed a multivariate Cox's regression analysis with these factors included in order to estimate the independent effect of the p53 on OS2 of VMBC. This estimated the independent effect of p53 on OS2 in VMBC. The median OS2 was 13 months. The median OS2 was 8 months for patients with p53-positive tumors and 13.5 months for patients with p53-negative tumors (p = 0.259, Figure 4). In the univariate analysis, tumor size and the number of organs involved were predictive of a poor OS2 (p = 0.070 and 0.000, respectively; Table 5). In the multivariate analysis, only the number of organs involved remained a significant predictor of poor OS2 (p = 0.009).
Comparison of p53 status with respect to OS2: p53-negative versus p53-positive.
OS2: Time from diagnosis of visceral metastasis to death. The Kaplan-Meier survival curve compares 29 p53-positive patients to 68 p-53 negative patients with respect to OS2 (time from diagnosis of visceral metastasis to death).
Discussion
Many data sets have shown that the absence of p53 mutations appear to predict a longer DFS and OS following primary therapy11,12,13,14,15,16,17,18,19. However, it remains unclear whether p53 has significance in the clinical course of VMBC and how it relates to other factors thought to influence the prognosis of VMBC. Hence, the rationale for this study is to evaluate and compare the role of p53 as a marker for prognosis and survival among VMBC patients. Consistent with previous findings, p53-positive VMBC was more likely to have an advanced TNM stage and mixed metastases at diagnosis than p53-negative breast cancer20. The p53 positivity rate for VMBC in this study (29.9%) was concordant with previous reports21. Also congruent with previous findings22,23,24, our study indicated that p53 was associated with tumor aggressiveness. In all the investigated patients, p53 expression correlated with a higher tumor stage and multiple organ involvement (visceral organ and bone). Patients who expressed p53 had a poorer DFS (P = 0.004) and OS1 (P = 0.021) than p53-negative patients. Then a question was raised. One reason for a shorter DFS in p53-positive breast cancer may be the relative resistance of p53-positive tumors to chemotherapy. Studies of women treated with a variety of chemotherapeutic regimens, or hormonal therapy, suggest that p53 status may be predicative of a response to therapy25,26. Retrospective analyses of randomized clinical trials, using either IHC measurement of p53 expression (CALB 9344) or TP53 gene sequencing (BIG 02-98), have identified a correlation between patients with a poor prognosis and those treated with adjuvant doxorubicin and cyclophosphamide, or doxorubin27,28. In addition, When TP53 mutations occur, it usually exert cancer-promoting effects, not only by dominant-negative inactivation of the remaining wild-type allele, but also through authentic oncogenic gain-of-function activities, which include a wide range of newly acquired oncogenic properties that are not found in the wild-type p53, such as increased genomic instability and cell proliferation, augmented invasion and metastasis and inhibition of apoptosis29,30. Taken together, all these may contribute to the poor clinical outcome of breast cancer with visceral metastasis. In concordance with these findings, we also found that p53-positive breast cancer was more likely to have an advanced TNM stage at the primary diagnosis, which may be related with the poor DFS and OS1. However, our study did not observe p53 had an impact on OS2. This is an interesting clinical phenomenon and has not previously been studied. Why this well recognized prognostic factor can lose its predictive value in VMBC is unknown and remains unclear. Perhaps a breakthrough at the gene and protein level comparing breast cancer patients before and after visceral metastasis will reveal this phenomenon. More research is needed to improve the knowledge of p53 in order to make optimal decisions for VMBC.
Furthermore, p53 expression appeared not to be associated with a high tumor grade of differentiation, estrogen and progesterone receptor negative status, or HER2 status. This was not consistent with previous studies9. Perhaps larger sample sizes are needed to confirm the observations and determine whether associations exist that may have been missed in smaller studies.
The p53 tumor suppressor protein, encoded by the TP53 gene, is a transcription factor that, when activated as part of the cellular stress response, regulates genes involved in cellular processes including the cell cycle, apoptosis and senescence31. In this study, p53 protein accumulation was assessed by immunohistochemistry (IHC). IHC was ideal because wild-type p53 protein is rapidly degraded, whereas TP53 mutations are often associated with the production of a stable protein that is detectable by IHC of the cancer cells32. Currently, IHC is the most commonly used modality for the evaluation of p53 mutations because sequencing of the p53 gene in all breast cancers would be expensive and time consuming for daily practice. Therefore, p53 evaluation by IHC is a practical tool to assess the prognosis.
Treatment for metastatic breast cancer (MBC) has evolved in the past 20 years because of the discovery of new and better tolerated therapies. Survival of patients with MBC has improved, with median survival now ranging between 18 and 30 months and 5-year survival between 23% and 30%33,34,35. However, MBC with visceral metastasis remains terrible for patients. Our study indicated that 5-year survival was 22.68% with a median survival time of 35 months. Bones are the most common site for the first metastasis in women with ER-positive and HER2-normal breast cancer, whereas basal-like and HER2-positive breast cancer is more likely to recur in visceral sites, including the central nervous system36. Our results provide evidence that targeting mutant p53 may serve as a promising therapeutic strategy in the treatment and prevention of visceral metastasis for partial breast cancer bearing p53 mutations. Studies have indicated that knockdown of mutant p53 by siRNA was able to induce G2-phase cell cycle arrest and apoptosis in human bladder cancer cells. Moreover, this strategy cooperated with cisplatin in the inhibition of cancer cells37.
Using Cox's model analysis, our study indicated that the number of metastatic sites and primary tumor size were associated with a poorer OS2.
This study had several limitations. First, the retrospective design made it difficult to compare the p53 status, treatment response and clinical course of the patients. Second, the small sample size may have limited the ability to derive statistically significant results and correct for differences in subgroup characteristics. Our studies did provide more information in the evaluation of p53's significance in VMBC.
In conclusion, these results indicate, by IHC, that patients with p53 protein accumulation appear to have shorter DFS and OS1 than those without p53 expression. However, when patients develop visceral metastasis, presence or absence of p53 is not a clinically useful tool to predict the survival time from metastasis to death. Larger prospective studies will be needed to confirm these findings.
Methods
Patients
This was a retrospective study including patients who were diagnosed with VMBC at the Cancer Hospital of Shantou University Medical College between January 2002 and December 2011. To identify patients for the analysis, the breast cancer database of the hospital was searched. The database contained information about all breast cancer patients between 2002 and 2011 (n = 2012). For the use of these clinical materials for research purposes, prior consents from the patients and approval from the Ethics Committees of the hospitals were obtained. AllAll patients lacked evidence of distant metastases (M0) at the time of surgery. 334 patients developed visceral metastasis as their first site during follow-up. We selected 97 consecutive patients with complete data for analysis. All patients had baseline assessments that included a complete blood count as well as renal and hepatic function tests. Imaging studies before treatment included chest X-rays, abdominal ultrasounds and radionuclide bone scans. Computed tomography (CT) scans of the chest, abdomen and pelvis were conducted when deemed necessary by the treating physician. Chest X-rays, abdominal ultrasounds and laboratory studies were repeated every 3 months. In all the patients, disease management consisted of surgery, adjuvant radiotherapy, systemic chemotherapy in a neoadjuvant and/or adjuvant setting and adjuvant hormonal therapy in hormone-receptor-positive patients. Additionally, all the patients underwent a total mastectomy and received an anthracycline-containing regimen either in the neoadjuvant or adjuvant setting. Histopathological data was documented from the original pathological reports and included tumor size, axillary lymph-node status, grading, ER status, PR status and HER2 status.
Visceral metastases were classified as either visceral alone (lung, liver, or brain) or mixed visceral (visceral organs and bone involvement). Chemotherapies in the metastatic setting included docetaxel, paclitaxel, gemcitabine and vinorelbine.
Histopathologic assessment
All of the histopathologic information used in the analysis was directly documented from the original pathology reports. Grading, tumor type, ER status, PR status and HER2 status had been routinely recorded at our hospital and the tests were performed on formalin-fixed, paraffin-embedded tumor tissue. Monoclonal mouse antibodies against the estrogen receptor alpha (clone 1D5; 1:200 dilution, DAKO, Denmark) and monoclonal mouse antibodies against the progesterone receptor (clone pgR636, 1:200 dilution, DAKO, Denmark) were used to stain the primary tumors. The percentage of positively stained cells was included in the pathology reports. The tumors were considered to be ER-positive and PR-positive if 10% or more of the cells showed positive staining. A polyclonal antibody against HER2 (A0485, 1:200 dilution, DAKO, Denmark) was used and HER2 status was noted in the pathology reports as negative (scores 0, 1+ and 2 +) or positive (scores 3 +).
Immunohistochemical staining for p53
Samples were fixed in 4% paraformaldehyde or 10% formalin and embedded in paraffin. 4 micron sections were cut and placed on silane coated slides for immunohistochemistry. The paraffin sections were dewaxed and pretreated in 0.01 M sodium citrate buffer (pH 6.0) and microwaved on high for 5 minutes and low for 15 minutes to unmask the tissue antigen. These sections were then incubated with 3% hydrogen peroxide for 10 minutes at room temperature to block endogenous peroxidase. Immunostaining was performed with anti-p53 antibody (dilution 1:200, mouse monoclonal to p53, M-0430, Shanghai Long Island Biotech Co., Ltd, China) at 4°C overnight. The sections were then incubated with the Supervision TM Universal (Anti-Mouse/Rabbit) Detection Reagent (HRP) (Cat. #: D-3004, Lab Vision Corporation, Fremont, CA, USA) for 30 minutes and counterstained with hematoxylin (Thor and J.F. Lara). A positive nuclear staining visual score of 10% or greater for invasive cancer cells was considered p53 positive38.
Statistical analysis
Data was analyzed using SPSS 17.0 software (Inc., Chicago, Ill., USA) and descriptive statistics were used to determine the patient's clinical characteristics. The chi-square test was used to compare categorical tumor features in the p53-positive and p53-negative groups. DFS was disease free survival, OS1 was the time from initial diagnosis of breast cancer to death and OS2 was defined as the time from detection of the first metastasis to death. Kaplan Meier and log rank tests were used for the analysis of OS1 and OS2. Multivariate analysis was evaluated by step wise forward Cox's regression analysis. The Fisher exact test was used to assess the association significance of p53 distribution in patients with visceral metastasis. For all the analyses, P value ≤ 0.05 was considered statistically significant.
References
Largillier, R. et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol 19 (12), 2012 (2008).
Nakopoulou, L. L. et al. Prognostic significance of the co-expression of p53 and c-erbB-2 proteins in breast cancer. J Pathol 179 (1), 31 (1996).
Lowe, S. W. et al. p53 status and the efficacy of cancer therapy in vivo. Science 266 (5186), 807 (1994).
Yamashita, H. et al. p53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res 8 (4), R48 (2006).
Rolland, P. et al. The p53 positive Bcl-2 negative phenotype is an independent marker of prognosis in breast cancer. Int J Cancer 120 (6), 1311 (2007).
Andersson, J. et al. Worse survival for TP53 (p53)-mutated breast cancer patients receiving adjuvant CMF. Ann Oncol 16 (5), 743 (2005).
Dai, M. S., Sun, X. X. & Lu, H. Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol Cell Biol 28 (13), 4365 (2008).
Alsner, J. et al. Heterogeneity in the clinical phenotype of TP53 mutations in breast cancer patients. Clin Cancer Res 6 (10), 3923 (2000).
Dookeran, K. A. et al. p53 as a marker of prognosis in African-American women with breast cancer. Ann Surg Oncol 17 (5), 1398 (2010).
Harris, L. et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25 (33), 5287 (2007).
Wiltschke, C. et al. Coexpression of HER-2/neu and p53 is associated with a shorter disease-free survival in node-positive breast cancer patients. J Cancer Res Clin Oncol 120 (12), 737 (1994).
Daidone, M. G. et al. p53 expression, DNA content and cell proliferation in primary and synchronous metastatic lesions from ovarian surface epithelial-stromal tumours. Eur J Cancer 32A (8), 1388 (1996).
Rosen, P. P. et al. p53 in node-negative breast carcinoma: an immunohistochemical study of epidemiologic risk factors, histologic features and prognosis. J Clin Oncol 13 (4), 821 (1995).
Elledge, R. M. et al. p53 protein accumulation detected by five different antibodies: relationship to prognosis and heat shock protein 70 in breast cancer. Cancer Res 54 (14), 3752 (1994).
Kroger, N. et al. Prognostic and predictive effects of immunohistochemical factors in high-risk primary breast cancer patients. Clin Cancer Res 12 (1), 159 (2006).
Gulben, K., Berberoglu, U., Cengiz, A. & Altinyollar, H. Prognostic factors affecting locoregional recurrence in patients with stage IIIB noninflammatory breast cancer. World J Surg 31 (9), 1724 (2007).
Linjawi, A. et al. Prognostic significance of p53, bcl-2 and Bax expression in early breast cancer. J Am Coll Surg 198 (1), 83 (2004).
Overgaard, J. et al. TP53 mutation is an independent prognostic marker for poor outcome in both node-negative and node-positive breast cancer. Acta Oncol 39 (3), 327 (2000).
Clegg, H. V., Itahana, K. & Zhang, Y. Unlocking the Mdm2-p53 loop: ubiquitin is the key. Cell Cycle 7 (3), 287 (2008).
Gao, Q. Z. et al. [Significance and expression of p53, p21(Cip1/WAF1) and Gadd45alpha in breast neoplasm tissues]. Zhonghua Yi Xue Za Zhi 92 (34), 2389 (2012).
Sawaki, M. et al. High prevalence of HER-2/neu and p53 overexpression in inflammatory breast cancer. Breast Cancer 13 (2), 172 (2006).
Wedin, R., Skoog, L. & Bauer, H. C. Proliferation rate, hormone receptor status and p53 expression in skeletal metastasis of breast carcinoma. Acta Oncol 43 (5), 460 (2004).
Domagala, W. et al. p53 protein and vimentin in invasive ductal NOS breast carcinoma--relationship with survival and sites of metastases. Eur J Cancer 30A (10), 1527 (1994).
Chang, J. et al. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer 97 (3), 545 (2003).
Borresen-Dale, A. L. TP53 and breast cancer. Hum Mutat 21 (3), 292 (2003).
Varna, M. et al. TP53 status and response to treatment in breast cancers. J Biomed Biotechnol 2011, 284584 (2011).
Lacroix, M., Toillon, R. A. & Leclercq, G. p53 and breast cancer, an update. Endocr Relat Cancer 13 (2), 293 (2006).
Rahko, E. et al. A mutant TP53 gene status is associated with a poor prognosis and anthracycline-resistance in breast cancer patients. Eur J Cancer 39 (4), 447 (2003).
Shapira, I., Lee, A., Vora, R. & Budman, D. R. P53 mutations in triple negative breast cancer upregulate endosomal recycling of epidermal growth factor receptor (EGFR) increasing its oncogenic potency. Crit Rev Oncol Hematol (2013).
Muller, P. A. et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 139 (7), 1327 (2009).
Brosh, R. & Rotter, V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 9 (10), 701 (2009).
Petitjean, A. et al. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 26 (15), 2157 (2007).
Lowe, S. W. et al. p53 status and the efficacy of cancer therapy in vivo. Science 266 (5186), 807 (1994).
Yamashita, H. et al. p53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res 8 (4), R48 (2006).
Lowe, S. W. et al. p53 status and the efficacy of cancer therapy in vivo. Science 266 (5186), 807 (1994).
Wedin, R., Skoog, L. & Bauer, H. C. Proliferation rate, hormone receptor status and p53 expression in skeletal metastasis of breast carcinoma. Acta Oncol 43 (5), 460 (2004).
Zhu, H. B. et al. Silencing of mutant p53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells. World J Surg Oncol 11, 22 (2013).
Lara, J. F. et al. p53 Expression in node-positive breast cancer patients: results from the Cancer and Leukemia Group B 9344 Trial (159905). Clin Cancer Res 17 (15), 5170 (2011).
Acknowledgements
This work was supported by funds from the Major State Basic Research Development Program (No.2011CB707705), National Natural Science Foundation of China (No. 30973377, No.31271068) and the Key Laboratory of Breast Cancer Prevention and Treatment of Guangdong Province, P.R. China.
Author information
Authors and Affiliations
Contributions
P.Y. contributed to the experiments and clinical data interpretation. C.W.D. discussed the results and analysed the data and wrote the manuscript conceived and designed the experiments. S.X.L. contributed to the statistical analysis. M.K. contributed to polishing the English to improve the quality of this manuscript. G.J.Z. supervised and directed the overall project. All the authors were involved in the discussions.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yang, P., Du, C., Kwan, M. et al. The impact of p53 in predicting clinical outcome of breast cancer patients with visceral metastasis. Sci Rep 3, 2246 (2013). https://doi.org/10.1038/srep02246
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02246
This article is cited by
-
Involvement of miR-214-3p/FOXM1 Axis During the Progression of Psoriasis
Inflammation (2022)
-
Clinicopathological and epidemiological significance of breast cancer subtype reclassification based on p53 immunohistochemical expression
npj Breast Cancer (2019)
-
Prognostic cancer gene signatures share common regulatory motifs
Scientific Reports (2017)
-
Quantifying intrinsic and extrinsic control of single-cell fates in cancer and stem/progenitor cell pedigrees with competing risks analysis
Scientific Reports (2016)
-
JARID1B modulates lung cancer cell proliferation and invasion by regulating p53 expression
Tumor Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.