Abstract
After the knock-out (KO) of α1,3 galactosyltransfease (Gal-T), the Hanganutziu-Deicher antigen became a major antigen of the "non-Gal antigen" that is implicated in subsequent xenograft rejection. For deletion of non-Gal antigen, we successfully produced zinc finger nuclease (ZFN)-mediated monoallelic/biallelic male and female CMP-N-acetylneuraminic acid hydroxylase (CMAH) KO miniature pigs: the efficiency of the gene targeting (41.7%) was higher when donor DNA was used with the ZFN than those of ZFN alone (9.1%). Monoallelic KO pigs had no integration of exogenous DNA into their genome, indicating that this technique would provide a new avenue to reduce the risk of antibiotics resistance when organs from genetically modified pigs are transplanted into patients. Until now, both monoallelic and biallelic CMAH KO pigs are healthy and show no sign of abnormality and off-target mutations. Therefore, these CMAH null pigs on the Gal-T KO background could serve as an important model for the xenotransplantation.
Similar content being viewed by others
Introduction
Since pigs have similar physiology to human, pigs are considered to be an important biomedical model. Specifically, pigs are considered as potential organ donors for humans because of their compatible organ size and physiology and short breeding time1,2. The potential of pigs providing organs for xenotransplantation was advanced when pigs were produced by somatic cell nuclear transfer (SCNT) using the genetic engineered cells. Considering the value of pigs as potential organ donors for human, it is not surprising that the first knock-out (KO) pigs were for the purpose of xenotransplantation1,2. These pigs had a disrupted α-1, 3-galactosyltransferase (GGTA1) gene, a key gene that synthesizes the enzyme that generates galactose α-1, 3-galactose (Gal-T) epitopes. Exposure of pig organs to human blood results in hyperacute rejection (HAR). The rejection is caused by the presence of the Gal antigen on the pig vascular endothelium and natural anti-Gal antibodies in human serum3,4. When pig organs or tissues are transplanted into the human body, the human IgM isotype of anti-Gal binds to the antigens on the pig tissues, which causes the activation of the complement cascade resulting in cell lysis5. When organs from the GGTA1 KO pigs were transplanted into a monkey, HAR was avoided as the organs lacked Gal-T epitopes6. These first KO pigs demonstrated that genetically modified pigs can be a great source of organs for xenotransplantation.
Although first barrier for xenotransplantation using pigs has been overcome by Gal-T KO pigs, there are still many immunological challenges before pigs organs can be used in humans. Unfortunately, there is limited progress on the research due to low efficiency in producing genetically modified pigs. Because of the poor efficiency, only a few genes have been modified in pigs7. Unlike mice, in pigs genetic modifications need to be made in somatic cells that are then used for SCNT due to the lack of embryonic stem cells. However, the frequency of gene targeting in somatic cells is extremely low8,9. Recent developments in zinc finger nuclease (ZFN) technology allow us to modify the genome with precision and high efficiency. ZFNs consist of a non-specific cleavage domain of FokI endonuclease with a zinc-finger domain specific for a target DNA sequence10. Designed ZFNs can induce double-strand breaks (DSB) at specific locations in the genome and these DSBs result in either random mutations through non-homologous end joining (NHEJ) or stimulation of homologous recombination (HR) if donor DNA is provided11. This technology is widely applied in rodents12 but there are only limited reports in pigs. Utilization of ZNFs in pig somatic cells were first shown in 201013 and KO pigs using ZFNs and SCNT were first reported by us14. The first pigs with a targeted endogenous gene were recently reported: heterozygous KO pigs for the peroxisome proliferator-activated receptor gamma (PPARγ) gene were generated using ZFNs15. The ZFNs were delivered into somatic cells with a plasmid containing Neomycin (Neo) resistance gene to select cells transfected with ZFNs. The efficiency of ZFNs was around 4%, much higher than an the average frequency observed in traditional gene targeting; one in every 104 to 107 cells16. Also, a biallelic knockout pig was produced by ZFN modification of somatic cells followed by SCNT to target GGTA1 gene17. In this paper, Gal-T null cells were counter-selected using flow cytometry as the Gal-T epitope could be recognized by a specific lectin and then two rounds of SCNT were performed to produce Gal-T null pigs. While the efficiency of ZFNs in genetic engineering of pigs has been reported, it has been limited to only a few genes.
Since production of Gal-T KO pigs by two groups in 20021,2, non-Gal antigens are considered to be the next xenoantigen involved in the rejection phenomenon. Specifically, N-glycolylneuraminic acid (NeuGc) is one of the non-Gal xenoantigens of secondary importance to GGTA118,19. Similar to GGTA1 gene, CMP-Neu5Ac hydroxylase (CMAH) is widely expressed on the endothelial cells of many mammals except humans (both of these genes are pseudo genes in humans) and this epitope is a potential porcine target for the anti-non-Gal antibody in humans as it is responsible for the expression of NeuGC, a key non-Gal antigen20,21. Previously, we reported that cloning and characterization of the full-length CMAH gene from the pig22 and reported that Gal-T deficiency in pigs increases sialyltransferase activities that potentially raise non-Gal xenoantigenicity23,24. This increase in non-Gal xenoantigenicity may permit development of strategies to prevent immunologic responses to those epitopes. If clinically acceptable immunosuppressive therapy cannot sufficiently inhibit the production of a specific anti-non-Gal antibody, an alternative approach is to delete or modify the non-Gal epitope in pigs by genetic engineering19. Eliminating CMAH gene expression in the pig is important to produce organ donors for xenotransplantation.
In general, targeting vectors used for gene targeting contain antibiotic resistance genes such as neomycin or hygromycin for in vitro screening process. Similarly, in most cases a plasmid containing an antibiotic resistance gene is used with ZFNs to select somatic cells that are successfully transfected with ZFNs. This strategy, utilizing antibiotic selection such as G418 selection, may limit the application of pigs to human organ transplantation because transplanted organs will end up having an antibiotic resistance. To delete the selection marker at the targeted locus, a previous study used a cre/lox cassette system25. However, considering that somatic cells have a limited lifespan in vitro, this is a challenging approach and the cells are likely to reach senescence after two rounds of the transfection and selection process. If we can develop a new method, which does not use a selection marker to screen KO somatic cells, in other words, no integration of exogenous DNA, this technique would provide a new avenue to reduce the risk of antibiotics resistance in transplanted organs from transgenic pigs after xenotransplantation. Here, we present a rapid single-step approach to generate a gene knockout in pigs using engineered ZFNs for pig-human xenotransplantation. The aim of this study was to establish a suitable transfection and selection approach to produce KO somatic cells and utilized them in generating genetically engineered pigs with SCNT technology.
Results
Design and construction of ZFN and ZFN activity
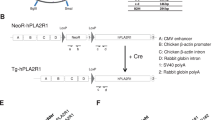
Custom ZFN plasmids were designed to bind and cleave exon 8 of the porcine CMAH gene. The design, cloning and validation of the ZFNs were performed by Sigma-Aldrich. In this study, two different strategies were performed to KO the CMAH gene in pig somatic cells; introducing ZFNs alone vs. ZFNs with donor DNA (Fig. 1a and b). Specifically, ZFNs will induce a DSB in the CMAH gene resulting in the generation of nucleotide insertions or deletions on the target, while ZFNs with the donor DNA will result in a targeting event by HR. To increase the frequency of the targeted cells with ZFN-induced mutations, we constructed a donor DNA vector. The donor DNA has a Neo resistance gene as a selection marker and contains two homologous arms for the HR reaction; both were less than 800 bp long. Fig. 1b shows a schematic design of the donor plasmid design. Donor plasmids were created corresponding to the cleavage location of the two ZFN pairs. ZFNs with confirmed activity in a yeast MEL-1 assay were obtained commercially from Sigma-Aldrich (Fig. 1c). In this study, a ZFN set, showed the highest activity in the yeast assay, were used. Next, the plasmids of the ZFN set were co-transfected with the donor DNA (providing a neomycin-resistance gene) into male or female porcine fibroblast cells by electroporation. Selecting cells with G418 allows for a significant enrichment of cells with potential CMAH KO cells (Fig. 1d).
Efficient targeting of CMAH locus by ZFNs.
(a, b) Strategy to disrupt CMAH gene expression. Diagram of (a) illustrates a part of exon 8 of the CMAH locus and the sequence recognized by the ZFN pairs. The ZFN target sequences are boxed. Each ZFN consisted of a five finger protein that recognized the target sequence (gray oval) and the nonspecific cleavage domain of the Fok I restriction enzyme. (b) The homologous sequence used to promote homologous recombination (arm 5′ and 3′). The middle diagram illustrates the donor DNA used to disrupt CMAH expression. A neo expression cassette (PGK-neo pA) has been introduced between the homologous arms to allow antibiotic selection. Lower diagram shows the edited CMAH locus after homologous recombination with the donor DNA. (c) ZFN with confirmed activity in a yeast MEL-1 assay. (d) A single cell selection after ZFN-alone or ZFN + donor DNA transfection. (e) Surveyor assay (Cel-I) nuclease assay for ZFN-induced mutations in CMAH gene. Cel-I endonuclease digest of the 534 bp heteroduplex DNA derived from the hybridization of the DNA from control and each targeted cells into 308 bp and 221 bp fragments proofed the mutagenesis of one CMAH allele (line 48 and 64) or bialleles (line 47 and 49) in the targeted cells. Arrowheads indicate the expected positions of the digested products. SM, E and K indicate size marker, control ear- and kidney-DNA amplified products, respectively. (f) Chromosome analysis in ZFN-targeted female (left) and male (right) somatic cells, which used for SCNT.
The surveyor mutation detection assay was performed to identify the frequency of targeted cells carrying a CMAH mutation by cutting heteroduplex DNA derived from hybridization of control pig DNA with DNA from ZFN introduced cells using the Cel-I endonuclease. The analysis revealed a partial cleavage of the 534 bp DNA into 308 bp and 221 bp fragments in a targeted cell (Fig. 1e). The chromosomal analysis shows the karyotype of the cells remained normal after all the selection processes (Fig. 1f).
Effects of the homology length of donor DNA in ZFN-mediated targeting efficacy
To identify the minimal homology length of donor DNA that can induce HR at the targeting locus, a series of DNA fragments with various lengths of the 5′ upstream or 3′ downstream region of CMAH exon 8 were cloned into PGK-neo + 2A+ GFP vector (Fig. 2). Each construct was transiently transfected into pig fibroblast cells along with the PGK-neo + 2A+ GFP plasmid to normalize transfection efficiency for the dual reporter assay. Pig fibroblast cells were recovered at 5 days after transfection with ZFN plasmids and donor DNA of various lengths. HR efficacy was analyzed by PCR and/or the number of GFP positive colonies. The best HR efficacy was obtained when donor DNA with 789 bp homology was used. However, the percentage of HR dropped to 50% for donor DNA with 200 bp homology compared to donor DNA with 789 bp homology. In addition, HR events were barely detectable when donor DNA with only 76 bp homology was used.
Comparison of homology length of donor DNA to induce HR in ZFN mediated gene targeting.
(a) Construction of donor vectors with 5 different homology lengths. (b) Quantitative PCR analysis for detection of ZFN/donor DNA mediated targeted cells. For donor DNA construct and PCR primer sets, see the Method. For PCR analysis of ZFN-assisted homologous recombination events, we used the same methods for screening and analysis of knockout colony using Neo A and CMAH B primers located outside of the recombination region (Supplementary table 1).
ZFN-driven targeted integration into an endogenous locus
Sixty-four and 48 neo-resistant colonies in male cells, transfected with the ZFN plasmid and mRNA, respectively, were analyzed by PCR. Nineteen colonies were identified as carrying a HR with the KO vector in the cells transfected with ZFN DNA. Fifteen colonies were confirmed as targeted in cells transfected with ZFN mRNA (Table 1). In the female cells, however, only three of 39 and two of 62 colonies transfected with the ZFN plasmid and mRNA, respectively, were positive. The frequency of gene targeting was higher in the male cells (30.4%) compared to the female cells (5%). Gene-targeting efficiency between the ZFN plasmid and ZFN mRNA was not different between male and female cells. However, gene-targeting efficiency using the ZFN-assisted HR of donor DNA showed dramatic differences between male and female cells. All KO cells were identified by PCR as heterozygotes with only one allele of the CMAH gene disrupted: only the expected 1.2 kb PCR products were obtained in the KO cells with successful targeting at the CMAH gene locus with donor DNA and ZFNs (Fig. 3c).
Selection of CMAH targeted cells using a selection-independent and -dependent zinc finger nucleases.
(a) Design of the donor DNA and primers used for this study. The locations of primers used for genotyping are shown in the figure. (b) SNP heterozygosity of the ZFN binding site on the CMAH gene. Sequencing PCR products include the ZFN cutting site show that there is a SNP within the ZFN binding site (arrow). Female show complete mismatch compared to the reference sequence of pig CMAH (NC_010449). The position of the DNA nucleotide mutation is indicated by the red box. (c) Screening of KO events by PCR. 1.2 kb PCR product using primers of Neo 3-1 and ScAS3 indicates a KO event. PCR products (1.2 kb) indicate the amplification of right HR junction using Neo3-1 and ScAS3 primer. M, size marker (λ/HindIII and 1 kb ladder); P, positive control; N, negative control; Number, G418-resistant colonies. (d) Mutagenesis generated by ZFN. Sequencing PCR products includes ZFN cutting site show that ZFN alone could generate mutations adjacent to ZFN cutting sites. An insertion of adenosine on the left side of the ZFN cutting site was observed. (e) Predicted amino acid sequence of CMAH from disrupted allele in KO pigs by NHEJ. Premature stop codon (*) is generated by NHEJ and series of amino acid sequence before the stop codon does not code for any known protein. Red letters indicate newly generated amino acids due to the frame shift.
SNP heterozygosity of the ZFN binding site on CMAH gene
Because targeting efficiency was distinctly different between male and female cells, we hypothesized that the difference in the targeting efficiency resulted from inconsistent ZFN binding affinity. Direct sequencing of PCR products from the male and female genomic DNA was conducted to confirm the ZFN binding site. ZFNs used in this experiment were designed based on accession number NC_010449 from NCBI. The direct DNA sequencing quantitation methods accurately quantitated SNP heterozygosity on the binding site of the left ZFN in male cells (Figure 3b). A Y peak (indicated by cytosine and thymine) can be observed on the left ZFN binding site of male cells. These results indicate that in male cells the cytosine on one allele was replaced with thymine. The cytosine was also mutated with a thymine in both alleles in the female cells. This analysis confirmed the presence of SNP at the ZFN binding site region and explain why the binding affinity of ZFNs is most likely decreased in female cells.
To further validate the idea that the ZFN mediated targeting was lower in females due to the SNP on the ZFN binding site region, fibroblast cells of Minnesota mini pigs with a different genetic background, whose genotype is identical to the reference sequence, were used for gene targeting. Here the targeting efficiency in male and female cells was similar (Table 2 and Fig. 3c). The average targeting efficiency of male and female cells was 41.7%. Biallelic modification from ZFN was similar in both males and females using the donor DNA; one incident each. These cells with biallelic modification were used to produce CMAH KO pigs.
Disruption of CMAH gene by NHEJ
When ZFNs designed for CMAH gene were introduced into male fibroblast cells without the donor DNA, a mutation from ZFN induced DSB was detected. After transfection, the cells were sorted into multiple 96-well plates; with a single cell in each well. A total of eleven cell colonies were screened and one colony showed targeted mutation of CMAH gene. The mutation was a single nucleotide insertion of adenosine adjacent to the ZFN cutting site. The targeting efficiency using ZFN alone was 9.1% (1/11 colonies; see Figure 3d for genotyping of the targeted event). Later, the donor cells were used to produce CMAH KO pigs and we observed the polymorphism in DNA sequence at the ZFN cutting site in the genome of the pigs.
Production of monoallelic and biallelic CMAH knock-out miniature pigs
After screening KO events from each colony derived from single cells, multiple cell colonies were used to produce CMAH KO pigs. Male C3 cells harboring a ZFN induced mutation through NHEJ on CMAH gene, male D11 and female D1 cells shown to have a biallelic modification of CMAH and the other cell lines had a monoallelic modification of CMAH were used to produce CMAH KO pigs. By using male C3 cells, SCNT-produced 13 piglets carrying a 1 bp insertion without insertion of donor DNA on one CMAH allele (Fig. 3d). Also, 5 miniature pigs carrying mono- or biallelic modification of CMAH gene were produced when ZFN with donor DNA was used for gene targeting (Fig. 4a and Supplementary Fig. 1). Genotyping DNA from cloned pigs showed the expected mutations in the CMAH gene. Genotyping from male C3 derived pigs, like donor cells, showed that there was an insertion of adenine adjacent to the expected cutting region of ZFN. Figure 3e shows the predicted amino acid sequence of the modified allele of CMAH KO pigs. The mutation caused the generation of a premature stop codon by a frame shift of the codon. When the newly predicted amino acid sequence was used to search for sequence similarity, none could be found (Fig. 3e). Using the female D1 colony, we were able to produce 2 pigs with biallelic mutations of the CMAH gene. The pigs from D1 showed mutations in both alleles that were apparently caused by a combination of both HR and NHEJ (genotypes of the pigs derived from male B2 and female D1 are shown in Fig. 4b). Sequencing of PCR products amplified from the HR junctions revealed that one allele was disrupted by a clear HR reaction and the other allele was modified by an insertion of a truncated form of the donor DNA; a partial Neo gene (around 500 bp) was detected (Supplementary Fig. 1a and c). In this study, the average efficiency of SCNT was 0.9% (Table 3) and, the CMAH KO pigs reached sexual puberty and are fertile with no sign of abnormalities (Fig. 4a).
(a) Images of CMAH KO pigs. (b) Genotyping of CMAH KO pigs. For genotyping, three different PCRs were run to verify KO events. Right indicates the amplification of right HR junction using Neo3-1 and ScAS3 primers. Left shows the amplification of left HR junction (1.2 kb for endogenous and 3.2 kb for KO by HR). Long is the the amplification of entire HR junction (1.8 kb for endogenous and 3.7 kb for KO by HR). PCR products from biallelic KO pigs suggest that one allele has 1 bp insertion through complete HR and the other allele has an insertion of 500 bp on the locus: lane 1 and 3: pigs from female D1 (biallelic KO); lane 2: a pig from B2 (heterozygous); lane 4: negative control. HR (red arrow), NHEZ (white arrow) and Endo (green arrow) indicate amplified DNA by homologous recombination, non-homologous end joints and endogenous DNA, respectively. (c) PCR amplification of FokI domain from genemic DNAs of CMAH KO pigs [41-1 and 47-1 are DNAs from male and female pigs shown in (a)]. HPRT gene was used as an internal control. (d) Upper) Cel-I digest of heteroduplex DNA revealed no additional off-target mutations at the 8 loci with highest homology to CMAH; lane 1, AGAP1; 2, TRPM7; 3, NJEJ1; 4, TLK2; 5, TMOD2; 6, ESR1; 7, AKAP13; 8, NME2, SM, size marker. Bottom) Genes, gene IDS and sequence homologies of CMAH related sequence to exclude off-target mutations. Upper case: ZFN binding sites; lower case; ZFN cut site; homolog base pairs in red. SM indicates size markers. (e) Western blot analysis: expression of CMAH gene in the fibroblast cells from wild type, CMAH monoallelic (MKO) and biallelic (BKO) mutant minature pigs; Actin was used as a housekeeping protein. (f) Comparison of Neu5Ac and Neu5Gc contents between control and KO pigs. The Neu5Gc content was determined based on the signal intensities (peak areas from Supplementary Fig. 2) of each 1,2-diamino-4, 5-methylenedioxybenzene (DMB) fluorescence–labeled Neu5Gc and Neu5Ac. Each value is the mean ± SD of triplicate determinations and was confirmed by t-test.
Since an antibody against mouse CMAH has no cross reactivity with pigs, synthetic peptides corresponding to amino acid residues (CQELVVEKDEENG for 5-terminal and KDPTDSKGIVEPPEGTK for 3-terminal) of porcine CMAH were used as immunogen to raise polyclonal antibodies against pig CMAH in rabbits. These affinity-purified antibodies specifically recognize pig C-terminal CMAH, as shown in Western blot analysis (Fig. 4e). The resulting analysis confirmed the loss of full length CMAH protein in the fibroblast cells derived from homozygous mutant pigs and a significant reduction in the CMAH protein in heterozygous pigs, while strong expression of CMAH was detected in control pigs.
Off-targeting analysis
There was no sign of integration of ZFN constructs in all the pigs produced during the study; no amplification of FokI sequence was detected from genomic DNA of the pigs (Fig. 4c and Supplementary table 3 for primer sets). Next, we tested the potential of off-site cleavage by the ZFN. No off-target mutation was seen in the 8 loci examined with highest sequence homology to the zinc-finger binding and cut site in the CMAH gene by the surveyor mutation detection assay (Fig. 4d and see the supplementary table 4 for PCR primer sets). As shown in Fig. 4d, these results strongly demonstrate that off-target mutations in CMAH KO pigs created by ZFN-mediated genome modifications have not occurred, demonstrating the high fidelity of the ZFN binding and activity.
Decreased transcript level of H-D and Tn-related genes in CMAH KO pig cells
Hanganutziu-Deicher (H-D) plays a pivotal role in acute immune rejection of pig xenografts23,24,26. Therefore, we examined the sialic acid (Sias) content by an intensity signal of chromatogram in fibroblast cells derived from control-, monoallelic (MKO)- and biallelic (BKO)-adult CMAH KO pigs using a calibration curve obtained for the DMB derivative of standard Neu5Ac and Neu5Gc (Fig. 4f and Supplementary Fig. 2). Unlike those of control and CMAH MKO pigs, we did not find any presence of NeuGC in fibroblast cells derived from CMAH BKO pigs.
Generally, H-D antigen families were classified as 2 different subfamilies; ST3Gal1, ST3Gal2, ST3Gal3, ST3Gal4, ST3Gal5, ST3Gal6 for ST3Gal, ST6Gal1 and ST6Gal2 for ST6Gal, according to the carbohydrate linkages synthesized27. To normalize the mRNA level of control-, monoallelic- and biallelic-pigs, actin mRNA was used as an internal standard. After normalization with actin mRNA, ST3Gal1, ST3Gal3 and ST6Gal1 gene expression in biallelic CMAH KO pigs were significantly down-regulated, whereas ST3Gal2, ST3Gal4 and ST6Gal2 in biallelic CMAH KO pigs were up-regulated, compared to those of control pigs, respectively (Fig. 5 and see the Supplementary table 5 for RT-PCR primer sets). This observation suggested that down-regulated ST3Gal1, ST3Gal3 and ST6Gal1 in biallelic CMAH KO pigs potentially should result in decreased Siaα2,3Galβ1,3GalNAc-R, Siaα2,3Galβ1,4GlcNAc-R and Siaα2,6Galβ1,4GlcNAc-R expression, but increased Siaα2,3Galβ1,3GalNAc-R, Sialyl Lew X:Siaα2,3Galβ1,4(Fucα1,3)GalNAc-R and Siaα2,4Galβ1,4GlcNAc-R expression, that act as an immune antigen within transplanted recipients, respectively. Also, ST6GalNac6 expression for Sialyl-Tn antigen, GalNT3 and GalNT7 expression for Tn antigen in biallelic CMAH KO pigs were significantly down-regulated compared with control pig (Fig. 5b, c).
Sialyltransferase gene expression levels in control, monoallelic and biallelic CMAH KO pigs.
(a) Electrophoretic analysis of RT-PCR from control, monoallelic and biallelic CMAH KO pig-derived fibroblast cells. (b) Comparison of sialyltransferase gene expression in control-, monoallelic- and biallelic-derived pig fibroblast cells by real-time RT-PCR. (c) Quantification of real-time RT-PCR analysis in control-, monoallelic- and biallelic-pig fibroblast cells. All RT-PCR reactions were conducted in triplicate and normalized with pig actin mRNA. Each of monoallelic- and biallelic-pig relative values is presented as an n-fold expression difference compared to the control pig, which was set as 1. *P < 0.05 and **P < 0.001.
Discussion
Here we demonstrate the feasibility of ZFN-mediated KO of the porcine CMAH gene and successful production of CMAH monoallelic and biallelic KO pigs by SCNT using the ZFN-targeted cells. Although potential application of transgenic pigs is well known, the practical application of genetically engineered pigs is hindered because of the inefficiency in their production. Those inefficiencies are a combined result of introducing genetic modification in somatic cells and subsequently making the pigs by SCNT. Here we address the efficiency of genetic modification of donor cells for application to xenotransplantation.
The Gal epitopes are the major xenoantigens that cause hyperacute rejection during pig-to-human xenotransplantation28. These Gal epitopes are also involved in acute vascular rejection of xenografts. A previous study indicated that organs from genetically engineered animals lacking the major xenoepitope Gal by KO of GGTA1 gene were remarkably protected from human complement-mediated injury, but that xenografted organs from pig to baboon ultimately died due to acute humoral xenograft rejection29. This observation clearly indicates that further development is necessary to control acute vascular and cellular rejection and chronic rejection of xenografts (e.g. by induction of tolerance). Carbohydrates such as Hanganutziu-Deicher (H-D), Thomsen-Friedenreich (T or TF), Tn and sialyl-Tn play a pivotal role in acute immune rejection of pig xenografts26. Among them, H-D antigens are glycoconjugate-bound N-glycolylneuraminic acid (Neu5Gc) which is a type of sialic acid such as N-acetylneuraminic acid (Neu5Ac). In the intracellular condition, Neu5Gc is mainly produced from Neu5Ac by catalyzing CMAH with cytochrome b5 and NADH as a cofactor26. Even though BKO pig-derived fibroblast cells did not showed any Neu5Gc expression, mRNA expression of H-D, T or TF, Tn and sialyl-Tn related genes in BKO-derived fibroblast cells was down-regulated or up-regulated compared to wild type (Fig. 5 and Supplementary Fig. 2). This is different from results using double KO mice where the expression of the genes was downregulated. This discrepancy could be derived by species or cell lineage specificity. Thus, these discrepancies might need further investigation.
In this study, we were able to generate CMAH KO cells by ZFN or ZFN with donor DNA although the efficiency of targeting event was higher when donor DNA was used with the ZFN; 9.1% versus 41.7%, respectively (Table 1). Also, no bialleic modification of CMAH was detected when ZFNs alone were used. A possible explanation for this difference is the difficulty of sorting out non-transfected from total cells used for the transfection. This explains why previous studies co-transfected ZFNs with a selection marker15,30. However, we were able to produce monoallelic CMAH KO cell line and pigs by just introducing ZFN constructs. We also efficiently obtained CMAH KO cells using ZFN-assisted HR in porcine fibroblast cells despite the fact that the KO vector consisted of a very short arm for HR (Fig. 2). To our knowledge, this is the first study showing gene targeting using ZFN-assisted HR of donor DNA in porcine somatic cells.
No significant differences were observed between ZFN plasmid and ZFN mRNA for targeting efficiency. In the first experiment using the targeting vector (donor DNA), the vector efficiently replaced the target sequences in male cells but not in female cells (Table 2) with no sign of biallelic modifications. This is in contrast to findings for ZFN-mediated deletion of GGTA1 gene in pigs, where no gender difference was observed for ZFN targeting of cells31. More detailed study revealed the presence of a SNP at the ZFN binding site. In male cells, the binding site for the left ZFN had a single base pair mismatch in one allele while in female cells, the mismatch was found on both alleles compare to NCBI reference sequence. The mismatch was likely responsible for the low targeting efficiency in the female cells compared to the male cells. This was further confirmed when we used fibroblast cells having the same genotype as reference sequence in NCBI for ZFN mediated gene targeting; a high efficiency of targeting was observed in both male and female cells (Table 2 and Fig. 3b). Furthermore, we could obtain biallelic KO in male and female somatic cells. Our findings highlight the importance of validating sequences of potential ZFN binding sites prior to the assembly of ZFNs. In addition, these results indicate that gene targeting by ZFN-assisted HR can occur in a gene that has SNP heterozygosity of the ZFN binding site; however, an identical match in ZFN recognition sequence is likely required for biallelic modification.
When we further investigated the length of homology required to utilize HR during ZFN mediated gene targeting, even a very small DNA fragment containing homology was able to induce HR. However, homology less than 100 bp in donor DNA resulted in the absence of HR during ZFN mediated gene targeting. In addition, we could find correlation between HR efficiency and length of homology in donor DNA; longer homology led to higher HR efficiency. Based on these findings we suggest that the homology should be at least over 100 bp to induce HR in ZFN mediated gene targeting using porcine somatic cells. Many researchers have used long homologous arms (6.8 – 21 kb) for recombination of the target sequence. Previous reports indicate that typically 200-800 bp homology flanking ZFN target sites are needed to induce HR in mammalian cells32. In flies, recommended total length of homology is 200–500 bp33. Our results show that the length of homology can be shorter (around 100 bp) than previously reported; however, longer homology can increase the frequency of HR events with donor DNA during ZFN mediated gene targeting. This information will be a useful parameter to build donor DNA that can be used in ZFN mediated gene targeting.
Utilization of ZFN in pig cells, first reported by transfecting ZFN mRNA into porcine somatic cells expressing the eGFP gene, resulted in ZFN-induced knockout of the target eGFP gene sequence13. Then we showed ZFN mediated targeting and SCNT could be used to produce pigs with specific genetic modifications14. In 2011 there was the first report of ZFN targeting an endogenous pig gene15, where heterozygous PPARγ KO pigs were reported. The efficiency was 4.2% but only 20% of pigs had disrupted PPARγ when the cells were used as donors for SCNT. In this study, we were able to genotype the targeted donor cells prior to SCNT and all pigs showed the expected genotypes. Recently, production of biallelic GGTA1 KO pigs was reported using ZFN and SCNT17. This was the first report of biallelic modification of an endogenous gene by using ZFN in pigs. The efficiency of biallelic targeting in the study was approximately 1% in the donor cells after ZFN transfection. As mentioned in the introduction, GGTA1 KO cells can be sorted by either lectin-based or drug selection methods and used for SCNT17,30,34. Therefore low efficiency in gene targeting may not be critical for animal production. However, there may be cases where such selection techniques are not practical or appropriate for a specific genetic modification. In our study the average biallelic modification using ZFN with donor DNA was 5.6%. Since we did not have a selection method in-place for CMAH KO cells, we identified CMAH KO mutants in donor cell colonies derived from single cells and produced KO pigs using those cells.
Previous studies of GGAT1 KO using ZFN produced the KO pigs by two rounds of SCNT17,30. The first round of SCNT was done using a pool of GGTA1 KO cells and then fetuses were retrieved from the first SCNT. After genotyping the fetuses, pigs were re-cloned from cells with the selected mutation. This is understandable as fibroblast cells are primary cultured cells with limited proliferation capacity. In this study, we were able to efficiently identify genetic modifications in fibroblast cells by ZFN thus allowing us to produce KO pigs by just one round of SCNT. Compared to previous studies using ZFN to produce KO pigs, we could produce CMAH KO pigs more efficiently. Efficient genetic modification of somatic cells for SCNT is very important to produce genetically engineered pigs. Here, we demonstrate that KO pigs can be efficiently generated by application of ZFN. We were able to produce CMAH KO pigs from KO cells derived by ZFN alone and ZFN with donor DNA, but the efficiency of targeting using donor DNA was higher. After the completion of this manuscript, we found a report describing production of CMAH KO pigs35. In this study, we have successfully generated both of male and female CMAH KO pigs. Both male and female cells showed the high fidelity of ZFN binding and activity and we also demonstrated an effective method to utilize a donor DNA during ZFN mediated gene targeting.
In conclusion, the methods in this study describe an approach for generating specific CMAH KO cell lines and pigs that can be used to test the long-standing question of why Gal-T KO-derived pig organs transplanted to baboons eventually result in acute rejection. In conclusion, we predict that these Minnesota miniature CMAH KO pigs will be valuable sources for pig to human xenotransplantation.
Methods
The study protocol and standard operating procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the South Korea National Institute of Animal Science (IACUC approval number: 2010-006, D-grade). And all animals used in the experiments were approved by the Institutional Animal Care and Use Committee of the University of Missouri.
Design and construction of ZFN and ZFN activity
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Defined fetal bovine serum (FBS), DMEM, non-essential amino acids and sodium pyruvate were purchased from HyClone (Logan, UT, USA). All restriction enzymes were purchased from Takara (Ohtsu, Japan). ZFNs designed to cleave the exon 8 region of the CMAH gene were purchased from Sigma-Aldrich. These ZFNs had a five-zinc finger protein recognizing 15 bases (Fig. 1a).
Construction of donor knockout vector
Homologous arms used for production of KO pigs were amplified by polymerase chain reaction (PCR) from Minnesota miniature pig genomic DNA. The 789 bp 5′ arm fragment of the CMAH gene was cloned by PCR amplification using a forward primer with non-priming XbaI site (TCTAGACTCTCTATTTGGTGGCTCTGTTT) and a reverse primer with an additional EcoRI site (GAATTCAGGAGTTTCTTCCTTTCTGTTTT). The 763 bp 3′ arm fragment was amplified using a forward primer with an additional XhoI site (CTCGAGCCTACAACCCAGAATTTACTGCC) and a reverse primer with an additional KpnI site (GGTACCAACAGGGACCTGCCAAGAGGCCA). PGK-neo-polyA fragment for positive selection was isolated from the pKJ2 neo plasmid with EcoRI and XhoI digestion. All the fragments for PCR amplification were subcloned into pGEM-T easy vector (Promega. Madison, WI, USA) and the sequences of all fragments were confirmed. To construct the KO vector (donor DNA), the PGK-neo-polyA fragment was ligated into the EcoRI and XhoI site of pBluescriptII SK-plasmid to produce the pBSK-PGK-neo-polyA plasmid. Then, the 789 bp 5′ arm fragment was inserted on the XbaI and EcoRI sites of the pBSK-PGK-neo-polyA plasmid to produce the pBSK-5′ arm-neo plasmid. Finally, to generate the KO vector, the 763 bp 3′ arm fragment was ligated into XhoI and KpnI of the pBSK-5′arm-neo plasmid (Fig. 1b). These KO vectors were linearized by digestion with the NotI restriction enzyme prior to transfection.
To construct the donor DNA with minimal homology sequence, the 5′ and 3′ arms (789, 240, 200, 160 and 76 bp) were cloned by PCR amplification using primer sets in Supplementary table 1. These primer sets contained NotI and EcoRI restriction enzyme site for the left arm and HindIII and XhoI restriction enzyme site for the right arm. The PGK-neo fragment (EcoRI-SalI) was amplified using pKJ2 plasmid as template by PCR with sense primer (GAATTCTACCGGGTAGGGGAGGCGC) and anti-sense primer with additional SalI sequence. The 2A sequence (92 bp) was isolated by XhoI and KpnI restriction enzyme of pBSK-2A sequence plasmid. The EGFP was cloned using pEGFP-N3 plasmid as template by PCR using forward primer with an additional KpnI site (GGTACCATGGTGAGCAAGGGCGAGGAGCTGT) and reverse primer with an additional HindIII site (AAGCTTTTACTTGTACAGCTCGTCCATGCCG) primer. The PGK-neo (EcoRI-SalI) was ligated into pBSK (EcoRI-SalI) vector to produce pBSK-PGKneo plasmid. The 2A sequence (XhoI-KpnI) was inserted into the SalI-XhoI site of pBSK-PGKneo plasmid to produce pBSK-PGKneo-2A plasmid. The EGFP fragment (KpnI-HindIII) was ligated into the KpnI-HindIII site of pBSK-PGKneo-2A plasmid to produce pBSK-PGKneo-2A-EGFP plasmid. To produce long and short arm knock-out vector, 5′ and 3′ arm fragments were inserted into NotI-EcoRI and HindIII-XhoI sites of the pBSK-PGKneo-2A-EGFP plasmid. For transfection of linearized knock-in vectors to porcine fibroblasts, knock-in vectors were digested with NotI enzyme.
Preparation of porcine ear fibroblasts and culture conditions
Pig ear fibroblast cells were prepared from ear skin biopsies from specific pathogen-free Minnesota male and female miniature pigs maintained at Seoul National University36. The fibroblasts were seeded on gelatin-coated dishes and plates (SPL Lifescience, Gyeonggi-Do, Korea) and cultured in DMEM, supplemented with 15% defined FBS, 1 × non-essential amino acids, 1 × sodium pyruvate, 0.1 mM â-mercaptoethanol, 100 units mL−1 penicillin and 100 μg mL−1 streptomycin in a humidified atmosphere containing 5% CO2 at 37°C.
Transfection of knockout vector, ZFN plasmid or RNA
Fibroblasts were cultured to 90% confluence and then washed with EDTA-PBS and treated with 0.25% trypsin-EDTA. After the fibroblasts were suspended with culture medium, the cells were isolated by centrifugation and then the cells were resuspended at 1.25 × 107 cells mL−1 in an F10 nutrient mixture for electroporation. Cell suspension (400 μL) was electroporated in a 4 mm cuvette with four 1 ms pulses using 400 V capacitive discharges using a BTX electro-cell manipulator (ECM 2001, BTX, Holliston, MA, USA). A total of 10 μg of linearized knockout vector and the circular ZFN plasmid or RNA was mixed in 100 μL of F10 nutrient mixture at a molar ratio of 1:1:1 (knockout vector: left ZFN: right ZFN) and used for electroporation. After electroporation, the cuvette was placed on ice for 10 min. The cells in the cuvette were re-suspended in 10 mL of medium, distributed to a 48-well plate and further cultured for 24 h. Antibiotic selection was performed for 11 days using 300 μg mL−1 of G418 (Gibco BRL, Grand Island, NY, USA). After selection, the single colonies were passaged in 24-well plates and further cultured for analysis by PCR (see the Supplementary table 2 for primer sets).
Screening and analysis of knock-out colony and pigs
Knockout colonies were identified by first and second PCRs. A 200 μL aliquot of cell suspension from a 24-well plate was recovered by centrifugation for the screening of G418-resistant colonies by the first PCR. Primers used to screen CMAH KO colonies are shown in Supplementary table 2. The cells were re-suspended in 50 μL of distilled water containing 0.05 mg/mL proteinase K (Roche, Indianapolis, IN, USA). To extract genomic DNA, the cells were incubated at 55°C for 130 min and heated to 98°C for 10 min to inactive proteinase K. The first PCR was performed in a 50 μL volume containing 20 μL genome extract, 0.1 M Neo 3-1 and CMAH ScAS3 primers located outside of the recombination region, 1 × PCR buffer, 1.25 U Ex Taq DNA polymerase (Takara) and 200 μM of each dNTP. PCR amplification was repeated for 35 cycles of 30 sec at 95°C for denaturation, 30 sec at 68°C for annealing and 2 min at 72°C for extension. Positive colonies from the first PCR were subcultured in 12-well and 6-well plates as well as 6-cm and 10-cm dishes, stage by stage. The cells were then frozen in the media (culture media containing 10% DMSO) for further analyses. Genomic DNA was isolated from the positive cells for the first PCR analysis using a GenElute Mammalian Genomic DNA Miniprep kit (Sigma-Aldrich) to accurately analyze the KO colonies by the second PCR. The second PCR amplification was conducted under the same conditions as the first PCR. Multiple HR junctions were amplified using ScS5/CMAHR and ScS5/ScAS3 primers (Supplementary table 2). The PCR products (20 μL) were confirmed by electrophoresis on a 0.8% agarose gel.
Analysis of the ZFN binding site
Genomic DNA was extracted from ear fibroblasts of male and female Minnesota miniature pigs using the GenElute Mammalian Genomic DNA Miniprep kit (Sigma-Aldrich). The PCR reactions were conducted with 100 ng genomic DNA, 200 μM of each dNTP and 1.25 units Ex Taq DNA polymerase, 1 × PCR buffer, 0.1 M sense (CTACTTCTGCATCACTCAACTGTCA) and antisense primer (AAAATAAGCTCCAGACCCCTACTAA) in a total volume of 50 μl. DNA was initially denatured at 98°C for 2 min and was then subjected to 35 cycles of 95°C for 30 s, 58°C for 30 s and 72°C for 1 min and a final extension step of 72°C for 15 min. The PCR products (377 bp) were electrophoresed on a 1.2% agarose gel and the amplified DNA fragments were extracted from the gel and purified using a QIAquick Gel Extraction kit (Qiagen. Valencia, CA, USA). Direct sequencing of both strands was performed using ABI PRISM 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA) and each electropherogram was visually analyzed using Chromas 2.13 (Technelysium Pty Ltd., Helensvale, Queensland, Australia).
No integration of ZFN construct into pig genome
To investigate the presence of ZFN construct in the genome, a fragment of FokI domain in ZFN vector and hypoxanthine guanine phosphoribosyl transferase (HPRT) gene was amplified by PCR. Genomic DNA from cultured cells derived from CMAH KO pigs were isolated as described previously. Fifty ng of genomic DNA was used for the PCR. PCR conditions for the amplification was initial denaturation for 2 min at 95°C followed by 32 cycles of 30 sec at 94°C for denaturation, 30 sec at 55°C for annealing and 30 min at 72°C for extension (see the Supplementary table 3 for PCR primer sets). Expected sized of the PCR products were 170 bp for FokI and 798 bp for HPRT. As a positive control ZFN plasmid was used for FokI and wild type pig DNA was used for HPRT. PCR products were loaded on 2.0% agarose gel.
Somatic cell nuclear transfer (SCNT)
For SCNT trials, sow-derived oocytes were purchased from ART (Madison, WI). The oocytes were collected from sow ovaries then shipped overnight in maturation medium (TCM199 with 2.9 mM Hepes, 5 μg/ml insulin, 10 ng/ml EGF, 0.5 μg/ml p-FSH, 0.91 mM pyruvate, 0.5 mM cysteine, 10% porcine follicular fluid, 25 ng/ml gentamicin). Upon arrival, the oocytes were transferred into fresh medium and cultured for additional 20–24 hours. After 40–42 h of maturation cumulus cells were removed from the oocytes by vortexing for 3 min in the presence of 0.1% hyaluronidase. The denuded oocytes were manipulated in the manipulation medium supplemented with 7.0 μg/mL cytochalasin B. The polar body along with a portion of the adjacent cytoplasm was removed and a donor cell was placed in the perivitelline space as previously described37. The reconstructed embryos were then fused in a fusion medium (0.3 M mannitol, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM Hepes) by two DC pulses (1-sec interval) at 1.2 kV/cm for 30 μsec (using BTX Electro Cell Manipulator, Harvard Apparatus, Holliston, MA). After fusion, fused embryos were fully activated with 200 μM thimerosal for 10 min in the dark followed by 8 mM dithiothreitol for 30 min38. Embryos were then incubated in PZM339 with 0.5 μM scriptaid, a histone deacetylase inhibitor, for 14–16 hours. The following day, the SCNT embryos were surgically transferred into the ampullary-isthmic junction of a surrogate at 0 or 1 day after observed estrus.
Antibody production and western blot analysis
Rabbit antiserum was raised by using synthetic peptides corresponding to amino acid residues (CQELVVEKDEENG for 5-terminal and KDPTDSKGIVEPPEGTK for 3-terminal) of porcine CMAH. For polyclonal antibody production, rabbits were injected with affinity-purified CMAH protein, initially with complete Freund's adjuvant and subsequently with incomplete Freund's adjuvant. Polyclonal antibodies from serum were purified using a Protein G Sepharose column (Amersham Pharmacia, Orsay, France) and tested for CMAH recognition by ELISA. The method of Kohler and Milstein40 was adapted to produce monoclonal antibodies. The isotypes of the selected monoclonal antibodies were determined using a commercially available isotyping kit (Roche Diagnostics, Mannheim, Germany). Western blot and sialic acid content analyses were designed and conducted as described previously39,19.
Analysis of sialic acid contents
A total of 0.2 mL of the WT, MKO, BKO-CMAH null pig cell lysate was mixed with an equal volume of 4 M propionic acid (pH 2.3). Sialic acids (Sias) were released by incubation at 80°C for 4 hrs according to a previous report41. The samples were cooled and centrifuged for 10 min at 20,000 g. The resulting pellets were resuspended in 0.5 mL of 0.1 M HCl, incubated for 1 hr at 80°C to completely release Sias from glycoconjugates and centrifuged as described above. The supernatants recovered after hydrolyses were passed through an Amicon Ultra-0.5, Ultracel-3 Membrane filter (Millipore, Bedford, MA, USA) and the total released Sias samples were lyophilized, resuspended in 10 μL of 2 M acetic acid and incubated at 55°C for 2 hr in the dark with 50 μL of a reagent consisting of 7 mM 1,2-diamino-4,5-methylenedioxybenzene (DMB), 0.75 M 2-mercaptoethanol and 18 mM NaHSO3 dissolved in water. Samples were cooled after derivatization and 10 μL aliquots were analyzed by HPLC on a reversed-phase ODS column (4.6 × 150 mm, TSK-gel column ODS-80TM; Tosoh Bioscience) with a 30% methanol mobile phase. The flow rate was maintained at 0.5 mL/min and the derivatized Neu5Ac and Neu5Gc were detected using a model RF-10A fluorescence detector (Shimadzu, Tokyo, Japan) at an excitation and emission wavelengths of 373 nm and 448 nm, respectively42. Quantification of Neu5Ac and Neu5Gc was performed by measuring the chromatographic peak areas for each sample and by using a calibration curve obtained for the DMB derivative of standard Neu5Ac and Neu5Gc (Sigma-Aldrich).
RNA isolation and real-time RT-PCR
Total RNA was extracted from WT, MKO and DKO CMAH-null pig fibroblast using a RNeasy mini kit (Qiagen, Valencia, CA, USA). Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was conducted using an ABI ViiA™ 7 system (Applied Biosystems, Foster City, CA, USA) and SYBR Green as the double-stranded DNA-specific fluorescent dye (Bio-Rad, Hercules, CA, USA) (see the Supplementary table 5 for RT-PCR primer sets). The pig actin gene was used as an internal control to normalize the RT-PCR efficiency and to quantify the expression of the genes in WT pig, heterozygote- and homozygote CMAH KO pig derived mRNA. After normalization with actin mRNA, we compared the relative expression of each mRNA in the heterozygote- and homozygote CMAH KO pig-derived genes with those of the controls. We performed RT-PCR on each sample independently and in triplicate.
References
Lai, L. et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295, 1089–1092 (2002).
Dai, Y. et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nature biotechnology 20, 251–255 (2002).
Bach, F. H., Turman, M. A., Vercellotti, G. M., Platt, J. L. & Dalmasso, A. P. Accommodation: a working paradigm for progressing toward clinical discordant xenografting. Transplantation proceedings 23, 205–207 (1991).
Cooper, D. K., Koren, E. & Oriol, R. Oligosaccharides and discordant xenotransplantation. Immunological reviews 141, 31–58 (1994).
Galili, U. Significance of anti-Gal IgG in chronic xenograft rejection. Transplantation proceedings 31, 940–941 (1999).
Kuwaki, K. et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nature medicine 11, 29–31 (2005).
Whyte, J. J. & Prather, R. S. Genetic modifications of pigs for medicine and agriculture. Molecular reproduction and development 78, 879–891 (2011).
Clark, A. J., Burl, S., Denning, C. & Dickinson, P. Gene targeting in livestock: a preview. Transgenic research 9, 263–275 (2000).
Laible, G. & Alonso-Gonzalez, L. Gene targeting from laboratory to livestock: current status and emerging concepts. Biotechnology journal 4, 1278–1292 (2009).
Le Provost, F. et al. Zinc finger nuclease technology heralds a new era in mammalian transgenesis. Trends in biotechnology 28, 134–141 (2010).
Urnov, F. D. et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435, 646–651 (2005).
Cui, X. et al. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nature biotechnology 29, 64–67 (2011).
Watanabe, M. et al. Knockout of exogenous EGFP gene in porcine somatic cells using zinc-finger nucleases. Biochemical and biophysical research communications 402, 14–18 (2010).
Whyte, J. J. et al. Gene targeting with zinc finger nucleases to produce cloned eGFP knockout pigs. Molecular reproduction and development 78, 2 (2011).
Yang, D. et al. Generation of PPARgamma mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell research 21, 979–982 (2011).
Carroll, D. Genome engineering with zinc-finger nucleases. Genetics 188, 773–782 (2011).
Hauschild, J. et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proceedings of the National Academy of Sciences of the United States of America 108, 12013–12017 (2011).
Morozumi, K. et al. Significance of histochemical expression of Hanganutziu-Deicher antigens in pig, baboon and human tissues. Transplantation proceedings 31, 942–944 (1999).
Miwa, Y. et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation 11, 247–253 (2004).
Basnet, N. B., Ide, K., Tahara, H., Tanaka, Y. & Ohdan, H. Deficiency of N-glycolylneuraminic acid and Galalpha1-3Galbeta1-4GlcNAc epitopes in xenogeneic cells attenuates cytotoxicity of human natural antibodies. Xenotransplantation 17, 440–448 (2010).
Tahara, H. et al. Immunological property of antibodies against N-glycolylneuraminic acid epitopes in cytidine monophospho-N-acetylneuraminic acid hydroxylase-deficient mice. Journal of immunology (Baltimore, Md. : 1950) 184, 3269–3275 (2010).
Song, K. H. et al. Cloning and functional characterization of pig CMP-N-acetylneuraminic acid hydroxylase for the synthesis of N-glycolylneuraminic acid as the xenoantigenic determinant in pig-human xenotransplantation. The Biochemical journal 427, 179–188 (2010).
Park, J. Y. et al. Alpha 1,3-galactosyltransferase deficiency in pigs increases sialyltransferase activities that potentially raise non-gal xenoantigenicity. Journal of biomedicine & biotechnology 2011, 560850 (2011).
Park, J. Y. et al. alpha1,3-galactosyltransferase deficiency in germ-free miniature pigs increases N-glycolylneuraminic acids as the xenoantigenic determinant in pig-human xenotransplantation. Cellular reprogramming 14, 353–363 (2012).
Akagi, K. et al. Cre-mediated somatic site-specific recombination in mice. Nucleic acids research 25, 1766–1773 (1997).
Ezzelarab, M., Ayares, D. & Cooper, D. K. Carbohydrates in xenotransplantation. Immunology and cell biology 83, 396–404 (2005).
Harduin-Lepers, A., Mollicone, R., Delannoy, P. & Oriol, R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology 15, 805–817 (2005).
Galili, U. The alpha-Gal epitope (Galalpha1-3Galbeta1-4GlcNAc-R) in xenotransplantation. Biochimie 83, 557–563 (2001).
Houser, S. L. et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation 11, 416–425 (2004).
Li, P., Estrada, J. L., Burlak, C. & Tector, A. J. Biallelic knockout of the alpha-1,3 galactosyltransferase gene in porcine liver-derived cells using zinc finger nucleases. The Journal of surgical research, (2012).
Hauschild-Quintern, J. et al. Gender non-specific efficacy of ZFN mediated gene targeting in pigs. Transgenic research 22, 1–3 (2013).
Chen, F. et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nature methods 8, 753–755 (2011).
Beumer, K. J., Trautman, J. K., Mukherjee, K. & Carroll, D. Donor DNA Utilization during Gene Targeting with Zinc-finger Nucleases. G3 (Bethesda, Md.), (2013).
Fujimura, T. et al. Production of alpha 1,3-galactosyltransferase gene-deficient pigs by somatic cell nuclear transfer: a novel selection method for gal alpha 1,3-Gal antigen-deficient cells. Molecular reproduction and development 75, 1372–1378 (2008).
Lutz, A. J. et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose alpha-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 20, 27–35 (2013).
Ahn, K. S. et al. Resurrection of an alpha-1,3-galactosyltransferase gene-targeted miniature pig by recloning using postmortem ear skin fibroblasts. Theriogenology 75, 933–939 (2011).
Lai, L. & Prather, R. S. Production of cloned pigs by using somatic cells as donors. Cloning and stem cells 5, 233–241 (2003).
Machaty, Z., Wang, W. H., Day, B. N. & Prather, R. S. Complete activation of porcine oocytes induced by the sulfhydryl reagent, thimerosal. Biology of reproduction 57, 1123–1127 (1997).
Yoshioka, K., Suzuki, C., Tanaka, A., Anas, I. M. & Iwamura, S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biology of reproduction 66, 112–119 (2002).
Shao, X., Tarnasky, H. A., Schalles, U., Oko, R. & van der Hoorn, F. A. Interactional cloning of the 84-kDa major outer dense fiber protein Odf84. Leucine zippers mediate associations of Odf84 and Odf27. The Journal of biological chemistry 272, 6105–6113 (1997).
Malykh, Y. N. et al. Regulation of N-glycolylneuraminic acid biosynthesis in developing pig small intestine. The Biochemical journal 370, 601–607 (2003).
Malykh, Y. N., Shaw, L. & Schauer, R. The role of CMP-N-acetylneuraminic acid hydroxylase in determining the level of N-glycolylneuraminic acid in porcine tissues. Glycoconjugate journal 15, 885–893 (1998).
Acknowledgements
This work was supported by Woo Jang-Choon project (PJ007849) from the Rural Development Administration (RDA), Republic of Korea and Food for the 21st Century at the University of Missouri. The authors would also like to acknowledge technical assistance provided by Jason Dowell, Lonnie Dowell, Keith Giroux and Lee Spate.
Author information
Authors and Affiliations
Contributions
D.N.K., K.L., M.J.K., J.H.K., Y.J.C., C.P., J.W. and A.B. performed vector design, cell transfection, surveyor mutation detection assay, pig CMAH antibody production and KO analysis. K.L., J.M., K.W.P., M.S. and C.M. performed embryo transfer, knock-out pig production and animal care. D.N.K., K.L., M.J.K., R.S.P. and J.H.K. wrote the manuscript. RSP and JHK supervised the project. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary data
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kwon, DN., Lee, K., Kang, MJ. et al. Production of biallelic CMP-Neu5Ac hydroxylase knock-out pigs. Sci Rep 3, 1981 (2013). https://doi.org/10.1038/srep01981
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01981
This article is cited by
-
A desirable transgenic strategy using GGTA1 endogenous promoter-mediated knock-in for xenotransplantation model
Scientific Reports (2022)
-
Human immune reactivity of GGTA1/CMAH/A3GALT2 triple knockout Yucatan miniature pigs
Transgenic Research (2021)
-
Molecular characterization of cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) associated with the erythrocyte antigens in dogs
Canine Genetics and Epidemiology (2019)
-
Intestine specific regulation of pig cytidine-5′-monophospho-N-acetylneuraminic acid hydroxylase gene for N-glycolylneuraminic acid biosynthesis
Scientific Reports (2019)
-
Use of gene-editing technology to introduce targeted modifications in pigs
Journal of Animal Science and Biotechnology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.