Abstract
The recent discovery of “black” TiO2 nanoparticles with visible and infrared absorption has triggered an explosion of interest in the application of TiO2 in a diverse set of solar energy systems; however, what a black TiO2 nanoparticle really is remains a mystery. Here we elucidate more properties and try to understand the inner workings of black TiO2 nanoparticles with hydrogenated disorders in a surface layer surrounding a crystalline core. Contrary to traditional findings, Ti3+ here is not responsible for the visible and infrared absorption of black TiO2, while there is evidence of mid-gap states above the valence band maximum due to the hydrogenated, engineered disorders. The hydrogen atoms, on the other hand, can undergo fast diffusion and exchange. The enhanced hydrogen mobility may be explained by the presence of the hydrogenated, disordered surface layer. This unique structure thus may give TiO2, one of the most-studied oxide materials, a renewed potential.
Similar content being viewed by others
Introduction
TiO2 has attracted substantial interest as a photocatalyst due to its activity for breaking water into oxygen and hydrogen under ultraviolet (UV) light irradiation1,2. However, its overall efficiency for solar-driven photocatalysis is very limited, because of its wide bandgap in the UV, which accounts for less than 5% of the total solar irradiation3,4. Much effort has been made to improve its optical absorption for its photocatalytic activity, with metal, non-metal, or self-doping3,4,5,6,7,8,9. The recent realization of black TiO2 nanoparticles10 has opened a new avenue to the long-wavelength optical absorption and triggered a great amount of interest11,12,13,14. However, our understanding of this new black TiO2 remains far from satisfactory. In this study, we attempt to improve our understanding of this black TiO2 nanoparticle by using comprehensive advanced characterization techniques together with ab-initio calculations. The results yield exciting insights on the properties of black TiO2 that may be applicable to other oxide nanomaterials.
It is well known that Ti3+ impurities and oxygen vacancy defects turn white TiO2 yellow or blue3,4,5,6,7,8,9,15 and it would be natural and reasonable to think that probably Ti3+ is also responsible for the black TiO2, as this material is obtained by reacting white TiO2 with hydrogen and in the bulk anatase phase, the presence of atomic hydrogen was found to yield Ti3+ centers21. Hydrogen adsorption was proposed to proceed through molecular H2 dissociation via a hydride intermediate and subsequent migration and studies of hydrogen adsorption on (TiO2)N clusters revealed that an initial hydrogen atom could easily bond to a terminal oxygen site13. The observed hydrogen diffusion into the TiO2 (110) surface with a barrier of approximately 1 eV suggests that hydrogen incorporation into the TiO2 lattice could take place at room temperature16,17,18,19,20. Depending on whether the crystal surface terminations are predominantly (001) or (101), hydrogen can penetrate the surface and be incorporated into the lattice easily16,22.
However, the black TiO2 here is actually different, in that no detectable Ti3+ was found based on our previous laboratory-source X-ray photoelectron spectroscopy (XPS) results10 and more experimental evidence shown herein. Furthermore strong evidence testifies that the additional valence band edge states in the black TiO2 which are believed to be responsible for the long-wavelength absorption of the black TiO2 go away when Ti3+ is induced by extended-period X-ray irradiation. In addition, we reveal important properties of the hydrogen and the disorder in the black TiO2, although a definitive conclusion is still under investigation. We believe that our study would shine more lights on this new material or the alike.
Results
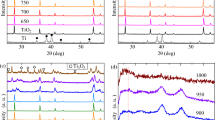
From high signal-to-noise ratio synchrotron X-ray spectroscopy, we found that both the post-hydrogenated black and pristine white TiO2 nanoparticles are free of Ti3+ species, as evident from the almost identical Ti 2p XPS spectra taken at 635 eV and the absence of Ti3+ feature ( Fig. 1 ). To examine the possible presence of impurities in the hydrogenated black TiO2, we performed XPS measurements as well as XAS at the C and N K-edges (Supplementary, Fig. S1). Both XPS and XAS spectra show similar spectral profile for the white and black TiO2, which essentially eliminates the possibility of an impurity contribution.
On the other hand, we found that the appearance of Ti3+ actually is accompanied with the disappearance of the additional valence band-edge states which are believed to be responsible for the long-wavelength absorption of the black TiO2 and thus the Ti3+ does not contribute to the additional valence band-edge states on the black TiO2. Fig. 1b shows that the hydrogenated black TiO2 exhibits additional electronic states above the valence band maximum of the white TiO2 nanocrystals10. These additional electronic states are believed to be responsible for the long-wavelength absorption of the black TiO2. Changes of XPS spectrum are observed upon an extended period (3 hours) of X-ray irradiation on the black TiO2. A small peak emerges at the high energy side of the Ti 2p XPS peak at 457.0 eV ( Fig. 1a ), while at the same time, the states above the valence-band maximum and characteristic of the black TiO2 disappear and the valence band XPS of the black and white TiO2 nanoparticles become identical ( Fig. 1b ). The Ti 2p XPS peak at 457.0 eV is characteristic of Ti3+ species. These findings provides the direct evidence that Ti3+ states are not responsible for mid-gap electronic states above the valence band of the hydrogenated black TiO2.
To further confirm the absence of Ti3+ in the black TiO2, we conducted other measurements in overcoming the limitation of the XPS technique, which normally only yields information about 1 nm deep. Soft X-ray XAS and X-ray emission spectroscopy (XES) have probe depths of tens of nanometers. Figs. 2a and 2b show the Ti L-edge and O K-edge XAS spectra of the black and white TiO2. The Ti L-edge XAS consists of contributions from L3(2p3/2-3d) (457–462 eV) and L2(2p1/2−3d) (462–467 eV) transitions23. The Ti L-edge absorption spectra again indicate no obvious sign of Ti3+ and almost identical absorption features in both black and white TiO2. The oxygen K-edge XAS spectra can be divided into a well-defined pre-edge region attributed to O 2p states with Ti 3d - O 2p mixing and the region above 536 eV, representing O 2p states hybridized with Ti 4s and 4p states. The similar O K-edge absorption spectra indicate that hybridization between Ti 3d and O 2p states in both the black and white TiO2 is extensive.
Figs. 2c and 2d shows the Ti L-edge and O K-edge XES spectra of the black and white TiO2. The Ti L-edge XES is attributed to the filling of the Ti 2p core hole from the Ti 3d, while the O K-edge emission comes from filling of an O 1s hole from the O 2p orbitals. Both relaxations are affected by the couplings between the Ti 3d and O 2p orbitals. Both black and white TiO2 show similar emission patterns excited at various energies, which suggests that the nature of the couplings in both black and white TiO2 is similar. The weaker peak in the Ti L-edge XES spectrum at the resonant excitation energy of 458.8 eV of the black TiO2 may indicate that the coupling between the Ti 3d and O 2p orbitals of the black TiO2 is slightly weaker due to the shared-bonding with the hydrogen and oxygen.
At this moment, by combining the information based on the almost identical Ti and O core-level XPS, soft X-ray XAS and XES results of black and white TiO2, we can conclude that the Ti3+ is not detected in black TiO2 within the technique accuracy and information depth employed in this study. This finding is also supported by the almost identical Ti K-edge X-ray near edge absorption spectra (XANES) of the black and white TiO2 (Supplementary, Fig. S2).
Discussion
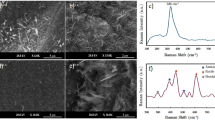
In order to get spatially-resolved information on the structural changes in the black TiO2, we performed high-resolution transmission electron microscope (HRTEM) studies of the black and white TiO2 nanoparticles. The white TiO2 is completely crystalline, displaying clearly-resolved and well-defined lattice fringes, even at the surface of the nanocrystal ( Fig. 3a ). The distance between the adjacent lattice planes is 0.352 nm, typical for anatase, uniform throughout the whole nanocrystal ( Fig. 3b and supplementary, Fig. S3). The black TiO2 nanocrystal has a crystalline-disordered core-shell structure ( Fig. 3c ). Structural deviation from the standard crystalline anatase at the outer layer is readily seen, where the straight lattice line is bent at the edge of the nanoparticle. While the core shows well-resolved (101) lattice plane with typical anatase plane distance, on the disordered outer layer, the distances between adjacent lattice planes are no longer uniform ( Fig. 3d and supplementary, Fig. S4). This structural difference would verify the disordered nature of the surface layer of the black TiO2. We prepared the grids for both samples under identical conditions and the samples were relatively stable under the beam during the measurements, which were done under the same conditions. Therefore, we believe that the observed differences between black and white TiO2 are characteristic of the materials, rather than artifacts of sample preparation or microscopy.
High-resolution transmission electron microscope (HRTEM) images and structural analysis of white and black TiO2 nanocrystals.
HRTEM (a) and line analyses (b) of white TiO2. HRTEM (c) and line analyses (d) of black TiO2. The zeros of the axis in (b) and (d) correspond to the left ends of the lines in (a) and (c). The red and green curves in (b) and (d) correspond to the red and green lines in (a) and (c).
In order to understand the role of hydrogen in the black TiO2, as the black TiO2 is obtained from reaction of white TiO2 with hydrogen, we first measured wide-range optical spectra from UV (200 nm) to the far-infrared (700 cm−1) of both black and white TiO2. The main difference between the optical spectra is in the visible and near-infrared regions from 400 to 1100 nm, where black TiO2 is optically absorbing ( Fig. 4a ). Both materials show similar absorption features from 1100 nm to 2500 nm (or 4000 cm−1) and both exhibit OH absorption bands near the 3400 cm−1 region, due to a bridging OH group ( Fig. 4b ). The peaks at around 3700 cm−1 are due to the O–H stretching and wagging modes. The strength of the terminal O–H mode is reduced after white TiO2 is converted to black TiO2, which suggests that the hydrogen incorporated into the TiO2 possibly does not passivate a significant number of O dangling bonds as this would otherwise increases the absorption. The wider OH absorption band of the black TiO2 indicates that the OH groups experience a more varied environment on the disordered black TiO2 surface than on the crystalline surface of the white TiO2. The linewidth difference may, in addition to the inhomogeneous effect referred to above, come in part from homogeneous (lifetime) broadening due to rapid exchange among sites.
Optical and vibrational spectra of white and black TiO2.
(a) Optical reflectance spectra of hydrogenated black TiO2 nanocrystals compared with white TiO2 nanocrystals. (b) FTIR reflectance spectra of hydrogenated black TiO2 nanocrystals compared with white TiO2 nanocrystals. (c)1H magic angle NMR spectra of hydrogenated black TiO2 nanocrystals compared with white TiO2 nanocrystals.
We further conducted 1H NMR measurements to examine the role of hydrogen in black TiO2 ( Figs. 4c ). Both the black and white TiO2 show a large peak at chemical shift of +5.7 ppm. The slightly larger linewidth in black TiO2 may be caused by the incorporation of H at bridging sites at the disordered phase produced during the hydrogenation process, or may be due to the bridging sites located on different crystallographic planes on the surface24. Compared to white TiO2, black TiO2 shows two additional narrow peaks at chemical shifts of 0.73 ppm and −0.03 ppm. These small and sharp 1H NMR resonances suggests that the hydrogen concentration is low and, there are dynamical exchanges between hydrogen in the different environments in black TiO2. Some examples of such dynamical exchange mechanisms are rapid isotropic diffusion and rapid exchange between similar proton environments25,26. These two peaks are probably associated with hydrogen located in the hydrogenated, disordered surface layer of the black TiO2 as a result of hydrogenation. Thus the presence of disordered surface layer of black TiO2 may provide an explanation for the enhanced hydrogen mobility in black TiO2.
To better understand the experimental measurement results of the hydrogenated black TiO2, we preformed ab initio DFT calculations on large TiO2 clusters. As shown in Fig. 5a , model A of Ti210O420H12 represents a normal white TiO2 nanocrystal of ~2.5 nm diameter where the dangling bonds of O atoms on the surface have been terminated by the H to form OH bonds. In model B, Ti218O436H70, additional H atoms have been introduced to simulate the hydrogenation process. After annealing and relaxation, the core of model B remains anatase-like, but its outer shell becomes disordered containing many distorted structures and the H atoms are partially bonded to O and Ti atoms, as shown as Fig. 5b .
ab initio DFT calculation results on large TiO2 clusters.
(a) model A structure of crystalline Ti210O420H12. (b) model B structure of disordered Ti218O436H70, Ti, O and H atoms are shown as grey, red and white balls, respectively. (c) The calculated electronic DOS of models A and B. The zero of the energy scale is set at the top of the valence band. (d) The calculated vibrational DOS of model B.
We plotted the calculated electronic density of states (DOS) of models A and B in Fig. 5c . Considering the fact that DFT underestimate the bandgap values, a scissor operator of 1.06 eV has been added to the conduction bands, by which the calculated bandgap of model a is calibrated to the experimental value of 3.30 eV and the calculated energy of mid-gap states have been linearly enlarged by a scaling parameter of 1.48 accordingly. Clearly model B results in the appearance of new states which extend from the valence band to more than 1.20 eV above the valence band maximum (VBM). There is also evidence of a small number of mid-gap states centered at about 1.80 eV above the VBM. The blue-shift of the VBM and reduction of the band gap agrees with the experimental XPS results and optical absorption in black TiO2. The DOS of model B shows a blueshift of the VB M by ~1.8 eV. In comparison with the 2.18 eV upshift of the VBM observed experimentally10, there is a difference of about 0.38 eV. While having much bigger size than model B, the real black TiO2 nano-clusters may contain more lattice distortions and with more mid-gap states to broaden their electronic DOS. From the vibrational DOS of H atoms in model B ( Fig. 5d ), the dynamics of hydrogen in black TiO2 can be divided into four frequency regions. The highest frequency modes around 4200 cm−1 are due to the stretching modes of the H–H bonds. That means some hydrogen atoms are trapped somewhere in the nanocrystal core or the disordered layer in the form of H2 molecules, but their amount is small10,27,28,29,30. These H-H stretching modes may not necessarily be infra-red active unless the local symmetry of the H2 molecules is not centro-symmetric. The modes at ~3000 cm−1 and ~1500 cm−1 come from the OH stretching and bending modes, respectively. Finally, the low frequency modes at around 500 cm−1 and below are produced by hydrogen atoms that are bound weakly to the disordered TiO2 lattice within the surface of model B. Unfortunately, these modes overlap with the phonon modes of TiO2 and as a result they may not be observed easily in the IR spectra. Since the hydrogen atoms are weakly bound they behave like free hydrogen and can diffuse readily from one site to another. This may account for the new sharp NMR peaks which appear in black TiO2 and have chemical shifts near zero ppm.
In summary, the rich physics of hydrogenated black TiO2 nanoparticles distinguish themselves from all known TiO2-based nanomaterials, white or coloured. Black TiO2 absorbs in the visible and infrared wavelength region, but unlike in traditional doped TiO2, Ti3+ defects or impurities are not responsible for the long-wavelength absorption of black TiO2. Hydrogen induces a disordered phase on the outside of crystalline anatase nanoparticle core. The hydrogen can move rapidly around and yield electronic structure changes from the alteration of the orbital overlapping in TiO2. These unique signatures would enable black TiO2 nanoparticles to improve the performance and efficiency of a range of solar energy systems, as already seen in a significantly enhanced solar-driven photocatalytic activity.
Methods
White TiO2 nanocrystals were first prepared with a precursor solution consisting of titanium tetraisopropoxide, ethanol, hydrochloric acid, deionized water and an organic template, Pluronic F127. The solution was heated at 40°C for 24 hours and then dried at 110°C for 24 hours. The dried powders were calcinated at 500°C for 6 hours to remove the organic template and enhance the crystallization of TiO2. The resulting white powders were first maintained in a vacuum for 1 hour and then hydrogenated in a 20.0-bar H2 atmosphere at about 200°C for 5 days to get black TiO2 nanocrystals. The UV-vis-NIR spectra were measured on a Cary 5000 UV-vis-NIR spectrometer. About 50 mg of TiO2 nanocrystals were pressed into the small sample container to make a flat surface and uniform density in a praying mantis diffuse reflection accessory. The FTIR spectra were measured on a Nicolet 6700 FT-IR Spectrometer with an attenuated total reflectance (ATR) unit. Small amount of the samples were pressed onto the ATR window for the measurements. All the measurements were conducted under ambient conditions in air. The baseline was corrected against air under the sample measurement parameters. The HRTEM images were taken on a Phillips CM 200 microscope. A small amount of sample was first dispersed in water by sonication. One drop of the aqueous suspension was then transferred onto a thin holey carbon film. The grids were then dried in an oven at 60°C overnight before TEM examination. The samples did not change during these preparations. The X-ray spectroscopic experiments were performed at the undulator beamline 7.0.1 of the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory (LBNL), with a spherical grating monochromator31. Ti 2p (L2,3) XES and O 1s (Kα) XES spectra were recorded by using a Nordgren-type grating spectrometer. The spectrometer was mounted perpendicular to the incoming photon beam in the polarization plane and the resolution was 0.3 eV and 0.4 eV, respectively, for Ti L- and O K-edge XES spectra. The monochromator was set to the excitation energies of 475 eV and 565 eV respectively, for Ti L and O K spectra with a resolution of 0.5 eV. The samples were mounted to have a beam incidence angle of 30° to the sample surface. For energy calibration of the Ti L- and O K-XES, the spectra of the reference samples Ti and TiO2 were measured. The base pressure of the chamber was 2 × 10−9 Torr. The absorption spectra at the Ti 2p and O 1s edges were measured by means of total electron yield (TEY) with a monochromator resolution set to 0.2 eV. The absorption intensity was normalized by the current from a clean gold mesh placed in the incoming beam to compensate for fluctuations of the incoming photon intensity. The XES and XAS spectra were brought to a common energy scale using an elastic peak in the emission spectra recorded at the excitation energy set at the absorption edge. The measurement depth of XAS as measured by TEY at these edges is normally around 10 nm and is around 100 nm for XES. Ti K-edge X-ray absorption measurements were performed in fluorescence mode at beamline 10.3.2 at the ALS32. Samples were spread on tape and spots with relatively low count rates were chosen in order to avoid the over-absorption ("self-absorption") effect. The probe depth for this method is about 5 μm, assuming the bulk anatase density. Spectra taken on different spots were consistent with each other. The edge for Ti foil was taken to be 4966.40 eV. The QuickXAS mode was used for data collection. Data were processed using standard software available at the beamline and from its website33. No radiation-induced effects were seen by comparing successive scans. We also performed high-resolution XPS and XAS experiments at the bending-magnet beamline 9.3.2 at ALS, LBNL34. The endstation can perform photoemission experiments up to a pressure of 1 torr. Incident photon energy can be tuned to adjust the kinetic energy of photoelectrons from the Ti 2p and O 1s core levels. The probing depth for the photon energies used in this study is about 1 nm. The energy resolution of the beam line is 3000. All NMR experiments were performed on an 8.45 tesla Oxford magnet with a three channel Tecmag Apollo NMR console, using a home-built, doubly tuned, MAS solid-state NMR probe with a 3.2 mm stator assembly from Revolution NMR. The magic angle spinning (MAS) rate was 12 kHz, the proton excitation pulse during the Bloch decay experiment was ~50 kHz and the spectra were externally referenced to adamantane. Each spectrum was acquired with 16 scans and the subtraction was performed after normalizing the peak at ~6 ppm. Both white and black TiO2 samples were evacuated at room temperature overnight to remove the surface adsorbed water vapor from the atmosphere.
All the theoretical calculations were based on the first-principles density-functional theory (DFT) using the Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation, with the Kohn-Sham equations solved with the projected augmented wave method as implemented in the VASP code35,36. All models were based on 30 × 30 × 30 Å3 supercells, where the atomic positions are relaxed until their residual forces are less than 0.05 eVÅ−1. The cut-off energy for the plane-wave basis set was 400 eV and the Brillouin zone was sampled with the single Γ–point.
References
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Grätzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Chen, X. & Burda, C. The electronic origin of the visible-light absorption properties of C-, N- and S-doped TiO2 nanomaterials. J. Am. Chem. Soc. 130, 5018–5019 (2008).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 107, 2891–2959 (2007).
Khan, S. U. M., Al-Shahry, M. & Ingler, W. B., Jr. Efficient photochemical water splitting by a chemically modified n-TiO2 . Science 297, 2243–2245 (2002).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271(2001).
Choi, W., Termin, A. & Hoffmann, M. R. The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 98, 13669–13679 (1994).
Anpo, M. & Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 216, 505–516 (2003).
Zuo, F. et al. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 132, 11856–11857 (2010).
Chen, X., Liu, L., Yu, P. Y. & Mao, S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011).
Naldoni, A. et al. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 134, 7600–7603 (2012).
Pan, H., Zhang, Y.-W., Shenoy, V. B. & Gao, H. Effects of H-, N- and (H, N)-doping on the photocatalytic activity of TiO2 . J. Phys. Chem. C 115, 12224–12231 (2011).
Syzgantseva, O. A., Gonzalez-Navarrete, P., Calatayud, M., Bromley, S. & Minot, C. Theoretical Investigation of the Hydrogenation of (TiO2)N Clusters (N = 1–10). J. Phys. Chem. C 115, 15890–15899 (2011).
Wang, G. et al. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 11, 3026–3033 (2011).
Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003).
Sun, C. et al. Hydrogen incorporation and storage in well-defined nanocrystals of anatase titanium dioxide. J. Phys. Chem. C 115, 25590–25594 (2011).
Kunat, M., Burghaus, U. & Wöll, C. The adsorption of hydrogen on the rutile TiO2 (110) surface. Phys. Chem. Chem. Phys. 6, 4203–4207 (2004).
Suzuki, S., Fukui, K. I., Onishi, H. & Iwasawa, Y. Hydrogen adatoms on TiO2(110)-(1 × 1) characterized by scanning tunneling microscopy and electron stimulated desorption. Phys. Rev. Lett. 84, 2156–2159 (2000).
Fujino, T., Katayama, M., Inudzuka, K., Okuno, T. & Oura, K. Surface hydroxyl formation on vacuum-annealed TiO2 (110). Appl. Phys. Lett. 79, 2716–2718 (2001).
Yin, X.-L. et al. Diffusion versus desorption: complex behavior of H Atoms on an oxide surface. ChemPhysChem 9, 253–256 (2008).
Di Valentin, C., Pacchioni, G. & Selloni, A. Reduced and n-type doped TiO2: nature of Ti3+ species. J. Phys. Chem. C 113, 20543–20552 (2009).
Islam, M. M., Calatayud, M. & Pacchioni, G. Hydrogen adsorption and diffusion on the anatase TiO2 (101) surface: a first-principles investigation. J. Phys. Chem. C 115, 6809–6814 (2011).
de Groot, F. M. F. et al. Oxygen 1s x-ray absorption of tetravalent titanium oxides: A comparison with single-particle calculations. Phys. Rev. B 48, 2074–2080 (1993).
Cracker, M. et al. 1H NMR spectroscopy of titania - chemical shift assignments for hydroxy groups in crystalline and amorphous forms of TiO2 . J. Chem. Soc. Faraday Trans. 92, 2791–2798 (1996).
Jonsen, P. Identification of different hydrogen-reduced titania crystallographis forms by 1H NMR spectroscopy. Catal. Lett. 2, 345–350 (1989).
Jonsen, P. A. 1H NMR Study of Reduced Ru/TiO2 and TiO2 . Colloids Surf. 36, 127–132 (1989).
Bavykin, D. V., Lapkin, A. A., Plucinski, P. K., Friedrich, J. M. & Walsh, F. C. Reversible storage of molecular hydrogen by sorption into multilayered TiO2 nanotubes. J. Phys. Chem. C 109, 19422–19427 (2005).
Lim, S. H., Luo, J., Zhong, Z., Ji, W. & Lin, J. Room-temperature hydrogen uptake by TiO2 nanotubes. Inorg. Chem. 44, 4124–4126 (2005).
Hu, X., Skadtchenko, B. O., Trudeau, M. & Antonelli, D. M. Hydrogen storage in chemically reducible mesoporous and microporous Ti oxides. J. Am. Chem. Soc. 128, 11740–11741 (2006).
Barnard, A. S. & Curtiss, L. A. Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 5, 1261–1266 (2005).
Nordgren, J. & Guo, J.-H. Instrumentation for soft X-ray emission spectroscopy. J. Electr. Spectrosc. Relat. Phenom. 110–111, 1–13 (2000).
Marcus, M. A. et al. Beamline 10.3.2 at ALS: a hard X-ray microprobe for environmental and materials sciences. J. Synchrotron Radiation 11, 239–247 (2004).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Grass, M. E. et al. New ambient pressure photoemission endstation at Advanced Light Source beamline 9.3.2. Rev. Sci. Instrum 81, 053106 (2010).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186(1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59 1758–1775 (1999).
Acknowledgements
This research has been supported by the Office of Energy Efficiency and Renewable Energy of the U.S. Department of Energy. TEM work was performed at the National Center for Electron Microscopy, which is supported by the Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy. The operations of the Advanced Light Source at Lawrence Berkeley National Laboratory are supported by the Director, Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy under contract number DE-AC02-05CH11231. X.C. thanks the support from College of Arts and Sciences, University of Missouri - Kansas City and University of Missouri Research Board. LL thanks support from the National Natural Science Foundation of China (No. 11174273) and the 100 Talents Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
X.C. and S.S.M. conceived the experimental research; L.L. and P.Y.Y. constructed the theoretical model. X.C. conducted UV-vis-NIR, FTIR and HRTEM measurements; L.L. and P.Y.Y. did the calculations; Z.L., M.E.G. and B.M. performed XPS measurements; M.A.M. conducted Ti K-edge XANES experiments; W.-C.W., P.-A.G. and J.G. carried out XAS and XES characterizations; and N.A.O. did NMR testing. X.C. and S.S.M. wrote the manuscript with contribution from all authors; everyone participated in discussions and analysis of the results.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Chen, X., Liu, L., Liu, Z. et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci Rep 3, 1510 (2013). https://doi.org/10.1038/srep01510
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01510
This article is cited by
-
Engineered disorder in CO2 photocatalysis
Nature Communications (2022)
-
Selective introduction of surface defects in anatase TiO2 nanosheets for highly efficient photocatalytic hydrogen generation
Rare Metals (2022)
-
Controlled hydrogenation into defective interlayer bismuth oxychloride via vacancy engineering
Communications Chemistry (2020)
-
Visible-light-driven photocatalysis for methylene blue degradation and hydrogen evolution reaction: a case of black TiO2 nanotube arrays
Journal of the Australian Ceramic Society (2020)
-
Robust electrochemistry of black TiO2 as stable and high-rate negative electrode for lithium-ion batteries
Materials for Renewable and Sustainable Energy (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.