Abstract
As an important component of the solid electrolyte interface in lithium ion batteries and an effective blanket breeding material in fusion reactor, the mechanical property of Li2O is of great interest but is not well understood. Here we show that the polycrystalline Li2O nanowires were formed in situ by touching and pulling lithium hydroxide under electron beam (e-beam) illumination. The Li2O nanowires sustained an enhanced elongation (from 80% to 176%) under low dose e-beam irradiation near room temperature as compared with that (from 51% to 57%) without e-beam irradiation. The extremely high deformability could be understood by the fast Li2O diffusion under e-beam irradiation and tensile stress condition. The large elongation without e-beam irradiation implies that nano-structured Li2O is ductile near room temperature.
Similar content being viewed by others
Introduction
As one of the simplest ionic oxides, lithium oxide (Li2O) is an important component of a solid electrolyte interface (SEI) layer in the anodes of lithium-ion batteries1 and an effective promoter for hydrogen storage materials2. The electrical and mechanical properties of the SEI layer determines performance and cycle lifetime of lithium ion batteries. Meanwhile, Li2O is the major product of the lithiated metal oxide anodes3,4. After lithiation, the pristine metal oxide electrodes will evolve to metal or lithium-metal alloy particles dispersed in Li2O matrix. As such, the mechanical properties of Li2O are critical for the integrity of the electrodes and will have impact on the cyclability of lithium-ion batteries.
More attractively, its high melting temperature (1711 K) and tritium breeding properties have made Li2O a demanding blanket breeding material in fusion reactor5, serving as a possible future energy resource for human beings. Nonetheless, how to utilize the nuclear energy safely has posed a challenge for the engineers in terms of designing appropriate materials working under the radiation environment, where the mechanical behavior was found to differ from the counterpart without radiation. For instance, Kiener et al indicated that the yielding strength of neutron-irradiated copper nano-pillars was affected by the interactions of dislocations with irradiation-induced defects6. In this light, a fundamental understanding of mechanical properties of Li2O with and without high-energy radiation is of significant value.
Besides, the perspective to reduce the critical feature sizes of modern electronic and mechanical devices has nourished the extensive investigation of nano-scale materials. As the sample dimension scales down to the nano-meter range, the mechanical behavior might be quite different as compared with that of bulk counterpart. For instance, recent work has revealed that the silicon7 and germanium8 nanowires (NWs) are indeed ductile while their bulk counterparts are usually brittle. Regarding the nano-scale oxides, the electron beam (e-beam) was shown to be a useful tool to effectively tune the mechanical properties. To be specific, the ductility of the SiO2 NWs can be greatly enhanced by low-intensity e-beam irradiation9. Furthermore, the e-beam irradiation may increase the Young's modulus of the zinc tin oxide NWs10. Nonetheless, the mechanical response of nano-structured Li2O, which would be meaningful for developing the nano-electrolytes and nano-electrodes for lithium ion batteries11, was rarely discussed. In this letter, we report a simple approach for fabricating the fresh polycrystalline Li2O NWs inside the transmission electron microscope. The corresponding mechanical behavior with and without e-beam irradiation has been investigated at room temperature.
Results
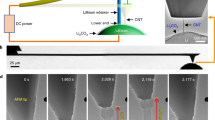
We found that the Li2O nano-structures could be synthesized by e-beam irradiation of lithium hydroxide (LiOH), confirming the previous experimental results12. The polycrystalline Li2O NWs were successfully fabricated by touching the LiOH surface followed by pulling with a gold tip (Fig. 1a). The NWs were fabricated as a result of the Li2O diffusion from the substrate to the gold tip as induced by the e-beam irradiation. Selected area electron diffraction (SAED) patterns indicate the substrate is a polycrystalline phase of LiOH (JCPDS 76-0911, Tetragonal, a = 0.355nm, c = 0.433nm) (Fig. 1b) and the extracted NW is Li2O (JCPDS 77-2144, Cubic, a = 0.462 nm) (Fig. 1c, see also Fig. S1). Figure 1d shows the electron energy loss spectroscopy (EELS) spectra of Li-K edge and O-K edge in LiOH (red curve) and Li2O (black curve). In LiOH, the Li-K edge peaks rise at 58.2, 62.7 and 75 eV and the O-K edge peaks rise at 529.4 and 535.1 eV; while in Li2O, the Li-K edge peaks appear at 58.2 and 62.7 eV and the O-K edge peaks appear at 526.1, 532.4 and 545.9 eV. The different near-edge fine structures of Li and O-K edges indicate the different electronic environment in LiOH and Li2O13. Subsequently, we conducted in situ tensile loading test of individual NWs with the whole deformation process directly recorded by video streaming.
In situ preparation of fresh Li2O NW from LiOH substrate.
(a) TEM image of the experimental set-up showing the fabrication of Li2O NW under the e-beam irradiation. SAED patterns showing the predominant phases of the substrate and extracted NW are polycrystalline (b) LiOH and (c) Li2O, respectively. The {200} diffraction ring with extremely low intensity is obscure in the SAED pattern. (d) EELS spectra of Li-K edge and O-K edge in LiOH (red curve) and Li2O (black curve) with the pre-edge background subtracted.
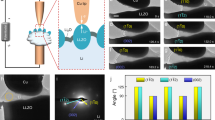
During the tensile loading tests, we intentionally decreased the beam current density to suppress the mass transport from the substrate to the NW root. Figures 2a-2e show a typical tensile loading test of a Li2O NW (see Supplementary Movies S1–S2 online) with the current density of 1.83×10−3 A/cm2. The initial length of the NW was estimated to be 5010 nm as shown in Fig. 2a. As we pulled, the segment between the two surface notches (pointed out by the arrowheads in Figs. 2b–2e), which exhibits a uniform diameter of around 323 nm, elongated significantly until a NW length of 13816 nm (Fig. 2e) was finally formed without breaking! Due to the limited range of the manipulator, we could not pull the NW any further. Hence, the elongation of ~176% is the lower bound. In addition, the necking process which reduced the diameter from the initial 323 nm (Fig. 2b) to 190 nm (Fig. 2e) was directly visualized. The strong diffraction contrast, which appeared and vanished quickly, was frequently observed during the necking process (pointed out by arrows in Figs. 2c and 2d), indicative of the massive dislocation activities during the NW elongation. In nanostructure materials, the grain boundaries (GB) and free surface might serve as the nucleation as well as the annihilation site of the dislocations, as demonstrated by both theoretical14 and experimental work15,16. Meanwhile, Figures. S2–S4 show three more examples signifying the large elongation of Li2O NWs under the e-beam irradiation with the strain rate of 4.1×10−3, 4.3×10−3 and 1.4×10−3 s−1, respectively. The total elongations are ~80% (Fig. S2), ~105% (Fig. S3) and ~110% (Fig. S4), respectively. During these tests, we have intentionally decreased the current density to the order of 10−4 A/cm2, which further suppressed the atomic diffusion process and thus lead to the smaller elongation as compared with that (176%) indicated in Fig. 2. The gradual necking process, which is a typical phenomenon during the tensile loading of ductile materials, continued until the fracture of NWs (Figs. S2–S4).
Super-elongation of a Li2O NW under e-beam irradiation.
(a–e) Sequential TEM images showing the elongation of Li2O NW under uniaxial tensile loading with a strain rate of 4.1×10−3 s−1. The time-lapse from (a) to (e) is 0, 279, 494, 829 and 1232 s, respectively. Arrowheads point to the surface notches while arrows mark the diffraction contrast induced by the dislocation activity.
To illustrate the e-beam irradiation effect on the deformation behavior, once the Li2O NW with a uniform diameter was formed, we blanked the beam (Fig. 3a) and then pulled the NW, taking images by intermittently turning on the beam for about 1 second per 3 minutes. A total elongation of ~51% was obtained (Figs. 3b–3c). It should be noted that the diffusion of Li2O from the substrate to the NW was negligible without beam irradiation as evidenced by the pretty stable reference point marked with arrowheads (Figs. 3a–3c). Another tensile test showing the elongation of ~57% in Li2O NW without e-beam irradiation is illustrated in Fig. S5.
Limited elongation of Li2O NW with the e-beam blanked.
(a–c) TEM images presenting the limited elongation of Li2O NW with the e-beam blanked during the tensile loading with a strain rate of 4.7×10−4 s−1. The time lapse from (a) to (c) is 0, 540 and 1080 s, respectively. Arrowheads point to the surface steps serving as the reference points.
Discussion
It is clearly seen the e-beam irradiation could effectively enhance the elongation of Li2O polycrystalline NWs. Without the e-beam irradiation, the plasticity of NWs could be mediated by the nucleation and subsequent annihilation of the dislocations at GB and free surfaces. Under the e-beam irradiation, besides the dislocation activities, the plasticity could be assisted by the fast atomic diffusion, promoted by dynamic displacements of the constituent atoms17. The basic idea is that the incoming electrons can be deflected by the Coulomb field of each atomic nucleus. The energy transferred from the incident electron to the atomic nucleus is given by18:


where θ being the deflected angle of the electron in the field of atom nucleus, Emax being the maximum energy that might be transferred, E0 being the incident electron energy (in eV) and A being the atomic mass number. Once the transferred energy exceeds the energy required for the displacement of the constituent atoms, the atom displacement may occur. In the current case, the maximum energy transferred from the 100 keV electron beam to lithium and oxygen are 80 and 15 eV, respectively. Since lithium and oxygen are both light element, it is believed that such high energies are able to create atom displacement and thus mass transport. This is actually the mechanism through which the fresh NWs were fabricated (the diffusion of the Li2O from the substrate to the gold tip). The fast diffusion assisted the annihilation of defects such as cracks nucleated during the mechanical loading and thus contributed to the plasticity.
Simultaneously, the current density applied to take the TEM images ranged from 3.2×10−4 to 0.1 A/cm2, several orders of magnitude lower than those employed previously in nano-engineering field19. Besides, the gold tip is a material with high thermal conductivity (300 W/mK) and is therefore able to conduct heat away quickly. The maximum temperature rise in Li2O NWs is thus expected to be less than 10 K18 (See the Methods for detailed information). As compared with its high melting point (1711 K), such small temperature rise would have little effect on its mechanical behavior. Moreover, the creep deformation, which may become noticeable at high temperature20, is thus not expected to be dominant during the current tensile tests.
It is worth noting that former works21,22 have demonstrated the formation of metallic lithium colloids in Li2O crystal irradiated by 1 MeV e-beam. To clarify the possible e-beam effect on the structure of Li2O NW, we have recorded in situ SAED patterns of single NW under long period e-beam irradiation (see Supplementary Movie S3 online). The patterns can be consistently indexed based upon pure Li2O polycrystalline phase (Fig. S1) as shown in Fig. 1c, implying that the Li colloids were not created under the low energy e-beam irradiation (i.e., 100 keV).
In summary, it has been found that the e-beam irradiation greatly enhances the ductility in polycrystalline Li2O NWs during the tensile loading which can be well understood by the fast diffusion caused by the e-beam irradiation under tensile stress. The large elongation (51% and 57%) without e-beam irradiation indicates that Li2O NWs is ductile and can sustain large mechanical stress, implying that the lithiated metal oxide nano-anodes in a lithium-ion battery might not be easily fractured during cycling. Our results have important implications for the application of Li2O nanostructures in nuclear environment and illustrate that e-beam irradiation can be an outstanding approach in nano-engineering field by means of tailoring the mechanical property in materials.
Methods
The experiments were carried out inside a FEI Tecnai F30 field emission gun transmission electron microscope (TEM) operated at 100 kV, with the Nanofactory TEM – scanning tunneling microscopy (STM) platform16. The LiOH crystals were attached to a gold rod by using electrically conductive, silver-filled epoxy-resin-based adhesive, CW2400 Circuit Works by ITW Chemtronics. Subsequently, the gold rod was inserted into one end of the TEM-STM platform. To successfully prepare a fresh Li2O NW inside the TEM, we firstly aligned the gold tip to touch the LiOH substrate. Subsequently, the contact area was irradiated by an e-beam with sizes ranging from 200 to 1000 nm (the corresponding current density spans from 0.5 to 12 A/cm2) to form the Li2O crystals in the substrate. The irradiation time is dependent on the current intensity. The higher current intensity, the less time required to nucleate the Li2O phase. Typically, for a current density of 12 A/cm2, the Li2O will be formed after 10 seconds e-beam irradiation. By slightly compressing the STM tip against the substrate, some of the Li2O crystals attached onto the STM tip. Finally, the tip was retracted back to form a Li2O NW which was subjected to further tensile loading (Fig. 1a).
The temperature rise under irradiation can be calculated by the Fisher's model23:

where I is the beam current, κ is the thermal conductivity, e is the electron charge (1.6×10−19 C), b is the sample radius, r0 is the beam radius and ΔE is the total energy loss per electron in a sample of thickness d. Since the energy loss in the sample is small compared with the initial energy which is 100 keV, the term ΔE/d is equal to the stopping power for electrons dE/dx which can be calculated from the Bethe-Bloch equation24:

in which Z is the atomic number of the target element, ρ is atomic density, ε0 is the dielectric constant (8.85×10−12 F/m), m is the electron rest mass (9.3×10−31 kg), υ is the electron velocity, c is the speed of light (3×108 m/s), E is the electron energy, Ie is the average excitation energy for electrons in the target and β = υ/c.
Currently, the acceleration voltage is 100 kV, thus, β = 0.548 and υ = 1.64×108 m/s. For Li2O, ρ = 4.04×1028 m−3 (mass density 2013 kg/m3), melting point 1711 K25, thermal conductivity κ = 7 W/mK26. The maximum temperature rise is estimated to be around 1K with I = 3.7 nA, b = 1.5 mm, r0 = 4 μm.
References
Liu, X. H. et al. Anisotropic Swelling and Fracture of Silicon Nanowires during Lithiation. Nano Lett. 11, 3312–3318 (2011).
Hu, Y. H. & Ruckenstein, E. Highly Effective Li2O/Li3N with Ultrafast Kinetics for H2 Storage. Ind. Eng. Chem. Res. 43, 2464–2467 (2004).
Huang, J. Y. et al. In Situ Observation of the Electrochemical Lithiation of a Single SnO2 Nanowire Electrode. Science 330, 1515–1520 (2010).
Etacheri, V., Marom, R., Elazari, R., Salitra, G. & Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243 (2011).
Mattas, R. F. & Billone, M. C. Materials for breeding blankets. J. Nucl. Mater. 233–237, 72–81 (1996).
Kiener, D., Hosemann, P., Maloy, S. A. & Minor, A. M. In situ nanocompression testing of irradiated copper. Nature Mater 10, 608–613 (2011).
Wang, L., Zheng, K., Zhang, Z. & Han, X. Direct Atomic-Scale Imaging about the Mechanisms of Ultralarge Bent Straining in Si Nanowires. Nano Lett 11, 2382–2385 (2011).
Smith, D. A., Holmberg, V. C. & Korgel, B. A. Flexible Germanium Nanowires: Ideal Strength, Room Temperature Plasticity and Bendable Semiconductor Fabric. ACS Nano 4, 2356–2362 (2010).
Zheng, K. et al. Electron-beam-assisted superplastic shaping of nanoscale amorphous silica. Nat. Commun. 1, 1–8 (2010).
Zang, J., Bao, L., Webb, R. A. & Li, X. Electron Beam Irradiation Stiffens Zinc Tin Oxide Nanowires. Nano Lett 11, 4885–4889 (2011).
Arico, A. S., Bruce, P., Scrosati, B., Tarascon, J.-M. & van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater 4, 366–377 (2005).
Hu, Y. & Ruckenstein, E. Nano-structured Li2O from LiOH by electron-irradiation. Chem. Phys. Lett. 430, 80–83 (2006).
Wang, F. et al. Chemical Distribution and Bonding of Lithium in Intercalated Graphite: Identification with Optimized Electron Energy Loss Spectroscopy. ACS Nano 5, 1190–1197 (2011).
Yamakov, V., Wolf, D., Salazar, M., Phillpot, S. R. & Gleiter, H. Length-scale effects in the nucleation of extended dislocations in nanocrystalline Al by molecular-dynamics simulation. Acta Mater. 49, 2713–2722 (2001).
Shan, Z. et al. Dislocation Dynamics in Nanocrystalline Nickel. Phys. Rev. Lett. 98, 095502 (2007).
Zheng, H. et al. Discrete plasticity in sub-10-nm-sized gold crystals. Nat. Commun. 1, 144 (2010).
Moore, N. W., Luo, J., Huang, J. Y., Mao, S. X. & Houston, J. E. Superplastic Nanowires Pulled from the Surface of Common Salt. Nano Lett. 9, 2295–2299 (2009).
Egerton, R. Radiation damage in the TEM and SEM. Micron 35, 399–409 (2004).
Xu, S. et al. Nanometer-Scale Modification and Welding of Silicon and Metallic Nanowires with a High-Intensity Electron Beam. Small 1, 1221–1229 (2005).
Yoshida, H., Ikuhara, Y. & Sakuma, T. High-temperature Creep Resistance in Rare-earth-doped, Fine-grained Al2O3. J. Mater. Res. 13, 2597–2601 (1998).
Vajda, P. & Beuneu, F. Electron radiation damage and Li-colloid creation in Li2O. Phys. Rev. B 53, 5335 (1996).
Prem, M., Krexner, G., Beuneu, F. & Vajda, P. Metallic colloids in lithium oxide after electron irradiation. Physica B 350, E999–E1002 (2004).
Fisher, S. B. On the temperature rise in electron irradiated foils. Radiat. Eff. Defects Solids 5, 239 (1970).
Jencic, I., Bench, M. W., Robertson, I. M. & Kirk, M. A. Electron-beam-induced crystallization of isolated amorphous regions in Si, Ge, GaP and GaAs. J. Appl. Phys. 78, 974–982 (1995).
Ortman, M. S. & Larsen, E. M. Preparation, Characterization and Melting Point of High-Purity Lithium Oxide. J. Am. Ceram. Soc. 66, 645–648 (1983).
Abou-Sena, A., Ying, A. & Abdou, M. Effective Thermal Conductivity of Lithium Ceramic Pebble Beds for Fusion Blankets: A Review. Fusion Sci. Technol. 47, 1094–1100 (2005).
Acknowledgements
J.W. would like to thank the financial support from 973 Program (2011CB933300), National Natural Science Foundation of China (51071110, 40972044, J0830310), China MOE NCET Program (NCET-07-0640), MOE Doctoral Fund (20090141110059) and the Fundamental Research Funds for the Central Universities. S.M. would like to acknowledge NSF CMMI 08 010934 through University of Pittsburgh and Sandia National Lab support. This work was performed, in part, at the Center for Integrated Nanotechnologies, a U.S. Department of Energy, Office of Basic Energy Sciences user facility. Sandia National Laboratories is a multi-program laboratory operated by Sandia Corporation, a Lockheed-Martin Company, for the U. S. Department of Energy under Contract No. DE-AC04-94AL85000. H.Z. would like to thank the Chinese Scholarship Council for financial support.
Author information
Authors and Affiliations
Contributions
H.Z. carried out the TEM experiments and wrote the paper. H.Z., Y.L. and S.M. contributed to the data analysis. J.Y.H. and J.W. revised the paper. The project was designed by J.W. and J.Y.H.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Movie 1
Supplementary Information
Movie 2
Supplementary Information
Movie 3
Supplementary Information
Supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zheng, H., Liu, Y., Mao, S. et al. Beam-assisted large elongation of in situ formed Li2O nanowires. Sci Rep 2, 542 (2012). https://doi.org/10.1038/srep00542
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00542
This article is cited by
-
Flexible welding of SiOx nanowire to macroporous carbon film and underlying new insights
SN Applied Sciences (2021)
-
Li metal deposition and stripping in a solid-state battery via Coble creep
Nature (2020)
-
Designing of metallic nanocrystals embedded in non-stoichiometric perovskite nanomaterial and its surface-electronic characteristics
Scientific Reports (2017)
-
In situ study of the mechanical properties of airborne haze particles
Science China Technological Sciences (2015)
-
Anelasticity of twinned CuO nanowires
Nano Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.