Abstract
Diatoms are generally known for superior mechanical properties of their mineralised shells. Nevertheless, many copepod crustaceans are able to crush such shells using their mandibles. This ability very likely requires feeding tools with specific material compositions and properties. For mandibles of several copepod species silica-containing parts called opal teeth have been described. The present study reveals the existence of complex composite structures, which contain, in addition to silica, the soft and elastic protein resilin and form opal teeth with a rubber-like bearing in the mandibles of the copepod Centropages hamatus. These composite structures likely increase the efficiency of the opal teeth while simultaneously reducing the risk of mechanical damage. They are supposed to have coevolved with the diatom shells in the evolutionary arms race and their development might have been the basis for the dominance of the copepods within today's marine zooplankton.
Similar content being viewed by others
Introduction
Copepods dominate the zooplankton in nearly all areas of the World Ocean and thus play a significant role in the pelagic food web1,2. Many copepod species feed mainly on phytoplankton and are important links between the primary producers and organisms of higher trophic levels. Accordingly, the knowledge of the impact of copepod grazing on phytoplankton stocks is essential for the understanding of particle and energy fluxes in the ocean.
Within the process of feeding, copepods use their mouthparts for the collecting, processing and uptake of food particles3,4,5,6. In this context, mandibular gnathobases play an important role as they are used to grip and, if necessary, mince the particles before ingestion. The morphology of the gnathobases has been shown to be related to the diet of the respective copepod species7,8. While carnivorous copepods possess gnathobases with rather long and pointed tooth-like processes (called ‘teeth’ in the following) at their distal end9, in species with a diet dominated by phytoplankton the gnathobases exhibit shorter and compact teeth8. As diatoms often account for a large proportion of the phytoplankton in many ocean areas2,10, these teeth regularly face mechanical interactions with diatom shells (also called frustules), which often are rather stable11.
Although detailed knowledge of the construction and material composition of the gnathobases is necessary to better understand the mechanical impact of grazing by copepods on the diatom frustules, only a few studies have addressed these important aspects so far. For some copepod species the presence of silica-containing teeth, so-called opal teeth, has been revealed12,13,14. However, the respective studies have focused on the elemental composition of the investigated structures only, while additional material differences have not been described.
The silica inclusions are hypothesised to increase the hardness and stiffness of the teeth and to have coevolved with the stable siliceous diatom frustules11. Structures consisting of hard and stable materials may easily break because of strong mechanical loads when being in contact with other hard structures15. Accordingly, it is important to analyse the material composition of the non-siliceous gnathobase parts to reveal information about the embedding and bearing of the opal teeth. These gnathobase parts might have evolved specific properties, which reduce wear and damage of the stable teeth. For detailed three-dimensional studies of the material composition of such arthropod structures the application of a combination of confocal laser scanning microscopy (CLSM) and autofluorescences emitted by different materials present in arthropod exoskeletons has proved to be very efficient16. Such analyses may be further improved by the use of fluorescence dyes specifically staining materials such as chitin and silica in the exoskeleton.
The present study aimed at revealing new insights into the morphology and the functioning of copepod gnathobases to improve the understanding of their adaptations to complex mechanical interactions between the gnathobases and compact diatom frustules. For this purpose, the above mentioned CLSM methods were applied in combination with bright-field light microscopy, scanning electron microscopy (SEM) and micro-particle-induced X-ray emission (µ-PIXE) to analyse in great detail the morphology and the material composition of gnathobases from females of Centropages hamatus. This species is distributed in coastal waters of the North Atlantic Ocean and adjacent seas17,18 and is one of the dominant copepods within the zooplankton of the southern North Sea19,20.
Results
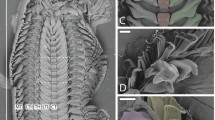
The mandibular gnathobases of Centropages hamatus possess a total of eight teeth (Fig. 1a). Compared to the other teeth, the ventral tooth and the first central tooth are considerably larger and, based on scanning electron micrographs, seem to consist of another material (Fig. 1a, c–e). Their surface exhibits a filamentous microstructure, which is not found on the other teeth (Fig. 1d–f). The single filament-like structures are oriented in longitudinal direction. A pronounced diastema exists between the two larger teeth. It is conspicuous that in many cases scratches are present in the surface material located at the diastema. These scratches were observed to be restricted to the areas, which are not covered with filament-like structures, while on the latter no scratches were found (Fig. 1c, d). In the ventral tooth a single tip is present, whereas all other teeth have two tips each.
(a, c–f) Scanning electron micrographs of mandibular gnathobases from female Centropages hamatus, cranial view: (a) overview of a whole gnathobase; (c) overview of the ventral part of the distal gnathobase structures; (d) detailed view of the ventral tooth shown in (c); (e) detailed view of the ventral tooth shown in (a); (f) filamentous microstructure on the surface of the ventral tooth shown in (c) and (d). (b) µ-PIXE mapping showing the distribution and concentration of silicon in a mandibular gnathobase of a female C. hamatus; the orientation of the gnathobase is similar to that of the gnathobase shown in (a). Arrows indicate areas with a large number of scratches. Scale bars = 20 µm (a), 10 µm (c), 5 µm (d, e), 1 µm (f); V = ventral tooth, C1 = central tooth 1.
The µ-PIXE analysis revealed the presence of silicon in the distal parts of the ventral tooth and the first central tooth (Fig. 1b). The results of the autofluorescence analysis, performed with CLSM, strongly indicate the presence of high concentrations of resilin, a protein found in the exoskeleton of many arthropods21, in single parts of the gnathobases (Fig. 2a). Additional autofluorescences were observed, however, they could not be clearly assigned to specific material sources as their origins have not been studied thoroughly so far. Furthermore, some structures such as the tips of the ventral tooth and the first central tooth exhibit only very weak autofluorescence, which does not allow for an effective visualization. Accordingly, the focus was laid on the visualization of the morphology and the material composition by using the autofluorescence of resilin only and combining it with the fluorescence of dyes.
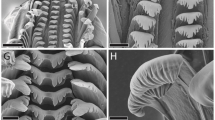
(a–f) Confocal laser scanning micrographs of mandibular gnathobases from female Centropages hamatus, cranial view ([a–c] maximum intensity projections [MIPs] showing the whole gnathobase; [d, e] 1 µm thick optical sections through the ventral tooth; [f] MIP showing the ventral and the first central tooth): (a) distribution of resilin; (b) chitinous exoskeleton (red) and resilin-dominated structures (blue); (c–f) chitinous exoskeleton (orange, red), resilin-dominated structures (blue, light blue) and silica-containing structures (green). (g–k) Bright-field micrographs of mandibular gnathobases from female C. hamatus stained with toluidine blue, cranial view: (g) overview of a whole gnathobase; (h, k) detailed view of the ventral tooth; (i) detailed view of the first central tooth and the smaller teeth in the central and dorsal parts of the gnathobase. Scale bars = 20 µm (a, b, c, g), 10 µm (f, h, i), 5 µm (d, e, k).
The results show that the ventral tooth and the first central tooth of the gnathobases have a complex internal morphology: on top of a chitinous socket a resilin-dominated (‘Resilin-dominated’ means ‘consisting of material with a resilin proportion, which is larger than the proportions of each of the other constituents’.) cap-like structure is located (Fig. 2b), which is covered by another cap-like structure containing silica (Fig. 2c–e). The filament-like structures, present on the surface of the latter, also contain silica (Fig. 2f). This result is in agreement with the scanning electron micrographs, which provide evidence for differences in the material composition between the two larger teeth and the rest of the gnathobase (see above). The other teeth mainly consist of chitin, however, the material at their tips is dominated by resilin (Fig. 2b, c). Besides the presence of resilin directly in the teeth, relatively large resilin-dominated structures were revealed at the dorsal edge of the central part and at the ventral edge of the proximal part of the gnathobases (Fig. 2b, c). These structures may vary in size and shape in gnathobases from different individuals (compare Figs. 2b and 2c).
The presence of resilin in the gnathobases, indicated by the occurrence of autofluorescence typical for resilin, was proved by staining with toluidine blue: in the distal part of the gnathobases the staining pattern was identical to the autofluorescence pattern. The two resilin-dominated cap-like structures within the larger teeth and the resilin-dominated tips of the smaller teeth were intensively stained (Fig. 2g–k). The structures with relatively high resilin concentrations present in the central and proximal parts of the gnathobases were weakly stained. All other structures were not stained at all.
The properties of the resilin autofluorescence observed in the gnathobases were compared to those of the resilin autofluorescences exhibited by the prealar arm (PA) and the wing hinge (WH) of the locust Schistocerca gregaria. These two structures are well-known for high concentrations of resilin21. The analyses of the emission spectra (Fig. 3) showed that the maxima of the resilin autofluorescences observed in the PA and the WH are located at 424 nm. Compared to this, the maximum of the resilin autofluorescence present in the gnathobases, located at 448 nm, is slightly shifted to longer wavelengths. The full width at half maximum (FWHM) of the emission spectrum obtained from the analysis of the resilin autofluorescence in the gnathobases is larger (108 nm, 418–526 nm) than those measured for the resilin autofluorescences in the PA and the WH (PA: 42 nm, 415–457 nm; WH: 46 nm, 414–460 nm).
Discussion
The general morphology of the mandibular gnathobases from Centropages hamatus resembles that of gnathobases traditionally classified as being typical for omnivorous copepods7,8. In fact, C. hamatus has been observed to feed on both phyto- and zooplankton and is thus considered omnivorous22,23,24. The two larger teeth at the ventral side of the gnathobases seem to be very suitable for grabbing larger food items such as large centric diatoms and for exerting punctiform pressure when crushing and mincing their frustules. This assumption is supported by the frequent presence of numerous scratches at the diastema, which mainly consists of chitinous material. The inclusion of silica in the tips of these larger teeth very likely enhances their hardness and stiffness, although the mechanical properties of the teeth have never been tested so far. However, the absence of scratches on the filament-like structures indicates that, compared to the chitinous material at the diastema, the silica-containing material in the teeth is harder and more resistant to damage caused by contact with mineralised food particles. It is imaginable that the longitudinally oriented filament-like structures on the surface of the tips further increase the stability, especially in the case of loads acting along the longitudinal tooth axis. Such loads are probably common during the crushing of stable food particles.
Diatom species, many of which have rather stable frustules11, may contribute significant proportions of the diet of C. hamatus25. In case the frustules are not crushed during the ingestion process, the diatoms may survive the passage through the guts of zooplankton organisms26. Accordingly, crushing of the diatom frustules during feeding is necessary to better digest the diatom cells and it is conceivable that the silica-containing teeth of the gnathobases may enable the copepods to more efficiently feed on and utilise diatoms. In some cases, while the copepods feed on diatoms, the pressure acting on the tips of the larger teeth might result in the exceeding of the breaking stress level and in an increased risk of crack-formation in and breakage of the teeth. A mechanical system, which has to resist high amounts of stress under pressure, will be more effective against damage and wear if it consists of a smart combination of materials with different properties (or a gradient in the material properties), since such an architecture leads to the minimisation of the probability of local stress concentrations27,28. As resilin is soft, flexible and elastic21,29, the resilin-dominated structures in the gnathobase teeth may function as flexible supports of the hard and stiff tooth tips. This may improve the resistance to mechanical damage while simultaneously providing the advantages of both stiffness and flexibility. For teeth of vertebrates, which have a comparable morphology with dentin, the teeth's major component, being covered by the five times harder enamel, it has been shown that a layered organisation with a gradient in the material properties is a key mechanism of the wear resistance28,30,31. With respect to the gnathobases, the structures with relatively high concentrations of resilin in the central and proximal parts may have a damping function making the whole gnathobases resilient and thus further reducing the risk of mechanical damage.
Nanoflagellates and chlorophytes, which are less stable and in most cases smaller compared to diatoms, may also be an important food source of C. hamatus23. When the copepods feed on these organisms, the smaller gnathobase teeth likely are more suitable for grabbing the food particles than the larger ones. The compliant resilin in the tips of these teeth may increase the grip of the teeth due to the formation of a larger contact area at the same applied load, very likely resulting in a more efficient grabbing of the food particles. The contact area and with it the grip of the smaller teeth is probably further increased by the presence of two relatively small tips. In the case of the larger teeth, which are supposed to be used to crush large and stable food particles, the presence of just one tip is advantageous as this facilitates the generation of a punctiform pressure. Even though the first central tooth contains two tips it can also be effectively used to create punctiform pressure as the morphology and the orientation of the tips is different from that of the smaller teeth. One tip is much longer than the second one so that in most cases only the longer tip gets contact to large undamaged food particles.
Although the emission spectra of the resilin autofluorescences observed in both the locust structures and the C. hamatus gnathobases are comparable to each other, the slight differences in the location of the maximum and the size of the FWHM indicate that the respective materials are not absolutely identical. Earlier analyses of the autofluorescences of resilin and isolated resilin compounds obtained from the PA and the WH of Schistocerca gregaria yielded comparable results: the emission spectrum and its maximum of the autofluorescence of native resilin was slightly shifted towards longer wavelengths compared to the autofluorescence properties of the isolated main autofluorescing resilin compounds dityrosine and trityrosine32. Accordingly, the small differences in the autofluorescence properties observed in the present study may be explained by differences in the overall material composition. It is imaginable that, compared to the resilin-dominated parts of the PA and the WH, the resilin-dominated structures in the gnathobases contain slightly lower concentrations of resilin and higher concentrations of other exoskeleton materials such as chitin, which is typically present in form of lamellae and fibres within the resilin structures21,33,34.
Plankton evolution is hypothesised to be mainly controlled by protection against specific ‘attack systems’ of grazers and predators having led to a large variety of morphologies and chemical and mechanical defence systems35. Diatoms, for example, have evolved the potential to reduce the reproductive success of copepods by the production of cytotoxic compounds36,37. In addition, their frustules often have complex architectures, which cause relatively high frustule stabilities and have very likely developed to resist pressure exerted on the frustules from outside11. Successful crushing of such mechanically protected frustules demands the deployment of rather specific feeding tools. In this context, the gnathobases of C. hamatus are an example of an adapted ‘attack system’ being capable of effectively capturing and processing food organisms with different sizes, shapes and mechanical protections by having combined diverse functional aspects. The rather complex morphology and material composition of the gnathobases have very likely coevolved with the food organisms. The described ‘attack system’ is supposed to represent a successful adaptation to the seasonal plankton succession typically occurring in the temperate North Atlantic Ocean, especially in its neritic zones where C. hamatus is abundant38,39,40,41. It may enable the copepods to effectively feed on both large and stable particles as well as small and soft ones with a simultaneous reduction of wear of the respective feeding structures. C. hamatus may thus react to the changing availability of single food taxa by switching to those taxa, which are dominant at a certain time.
In conclusion, this study indicates that the morphology and the material composition of copepod gnathobases are much more complex than previously shown. Up to now, the presence of high proportions of resilin has mainly been described for arthropod locomotory systems, where resilin provides e.g. high flexibility of joints, elastic energy storage within tendons and resistance to fatigue in wing folds21,33,42,43,44. The present study reveals for the first time a yet unknown function of resilin-dominated structures as soft and elastic supports of hard and stable biomineralised structures representing adaptations against mechanical wear and damage, which have very likely coevolved together with the advanced architectures of the food organisms in the evolutionary arms race. It is imaginable that the development of these complex composite gnathobase structures has been the basis for the dominance of the copepods observed today within the marine zooplankton.
Methods
Copepod sampling and gnathobase dissection
Zooplankton was sampled from aboard the research motorboat ‘Aade’ by vertical hauls at the station of the Helgoland Roads time series19,45 located between the two offshore islands of Helgoland in the German Bight (North Sea). Plankton nets with mesh sizes of 75 µm and 280 µm were used. The live samples were transported to the lab where female Centropages hamatus specimens were separated from the samples using a stereomicroscope (Olympus SZX9, Olympus Europa Holding GmbH, Hamburg, Germany) and glass pipettes. The live copepods were frozen within seawater at −70°C. For the analytical work they were defrosted and transferred to distilled water. Afterwards, the gnathobases were dissected from the copepods using a stereomicroscope (Wild M8, Leica Microsystems GmbH, Wetzlar, Germany) and fine preparation needles.
Scanning electron microscopy imaging
23 fresh gnathobases were dehydrated in a series of ethanols (Carl Roth GmbH & Co. KG, Karlsruhe, Germany; 70 %, 80 %, 90 %, 96 % and two times 100 %, 15 min each) and dried for 12 h in an exsiccator using hexamethyldisilazane (HMDS, Carl Roth GmbH & Co. KG)8. Conductive and adhesive carbon pads (PLANO GmbH, Wetzlar, Germany) were glued on aluminium stubs (PLANO GmbH) and the specimens were mounted on them using a stereomicroscope (Wild M8, Leica Microsystems GmbH) and fine preparation needles. Afterwards, they were sputter-coated with a 20 nm thick gold-palladium (80/20) layer using the high vacuum sputter coater Leica EM SCD500 (Leica Microsystems GmbH). The preparations were visualized with a Hitachi S-4800 field emission scanning electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan) at accelerating voltages of either 5 kV or 10 kV and an emission current of 10 µA applying either the upper (detection of high-angle secondary electrons) or the lower (detection of low-angle secondary electrons) detector.
Micro-particle-induced X-ray emission analysis
Three fresh gnathobases were dehydrated and dried as described above, but instead of HMDS 1-Propanol (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was used. During the drying process HMDS sometimes leaves tiny residues at the specimens, which might interfere with the results of element analyses (especially in our case, when analysing the presence and concentration of silicon, as HMDS contains this element). By contrast, 1-Propanol evaporates without residues while yielding reasonable drying results, which are much better than the results of air-drying. The dried specimens were transferred to a 0.9 μm thin polyethylene terephthalate film using a stereomicroscope (Wild M8, Leica Microsystems GmbH), adhesive labels (PLANO GmbH) and fine preparation needles. The µ-PIXE analysis of the silicon distribution and concentration within the gnathobases was performed at the Leipzig high-energy ion nanoprobe LIPSION as described earlier46.
Confocal laser scanning microscopy imaging
25 fresh gnathobases were checked for autofluorescences using different excitation wavelengths and emission filters16. In addition, two fluorescence dyes were used to visualize materials known to be present in the gnathobases. At first, the chitinous structures of the fresh gnathobases were stained with Congo red (Sigma-Aldrich Chemie GmbH)47. Subsequently, the gnathobases were thoroughly washed in distilled water and the silica-containing structures were stained with fluorescein isothiocyanate (Sigma-Aldrich Chemie GmbH) bound to (3-aminopropyl)triethoxysilane (Sigma-Aldrich Chemie GmbH) (FITC-APS48) at 4°C in a dark fridge for 12 h. After a second intense washing in distilled water the stained gnathobases were transferred to glycerine (≥99.5 %, free of water, two times distilled, Carl Roth GmbH & Co. KG) and mounted on object slides with reinforcement rings (Herlitz PBS AG Papier-, Büro- und Schreibwaren, Berlin, Germany) and high-precision cover slips (thickness = 0.170 mm +/− 0.005 mm, refractive index = 1.52550 +/− 0.00015, Carl Zeiss Microscopy GmbH, Jena, Germany)16,47. The specimens were visualized using the confocal laser scanning microscope Zeiss LSM 700 equipped with the upright microscope Zeiss Axio Imager.M1m (Carl Zeiss Microscopy GmbH) and four stable solid-state lasers (wavelengths: 405 nm, 488 nm, 555 nm, 639 nm). Micrographs of the different autofluorescences and the Congo red fluorescence were obtained applying a 40x objective (Zeiss Plan-Apochromat, oil immersion [immersion oil from Carl Zeiss Microscopy GmbH, refractive index = 1.518], numerical aperture [NA] = 1.3), all laser excitation wavelengths available, the respective emission filters and optimal settings as described earlier16. Structures stained with FITC-APS were visualized using an excitation wavelength of 488 nm and either a longpass (≥490 nm) or a bandpass (490–555 nm) emission filter. The final micrographs were adjusted for contrast, brightness and tonal values using the software packages Nikon Capture NX 2 (Nikon Corporation, Tokyo, Japan) and Adobe Photoshop CS4 (Adobe Systems, San José, California, USA).
Toluidine blue staining
Resilin in ten fresh gnathobases was stained with toluidine blue (Waldeck GmbH & Co. KG, Division Chroma, Münster, Germany; 5 mg per 100 ml of 0.1 M PBS buffer [pH = 7.4, Carl Roth GmbH & Co. KG]21) for 12 h. Glycerine effectively removes all excess toluidine blue. Therefore, after the staining the gnathobases were kept in glycerine for five days in order to completely remove the excess dye. Subsequently, the gnathobases were embedded in glycerine and mounted on object slides as described above and bright-field micrographs were taken using two different microscope systems: 1) the stereomicroscope Leica M205 A in combination with a 1.6x PLANAPO objective, the digital camera Leica DFC420 and the software Leica Application Suite 3.7 (Leica Microsystems GmbH); 2) the microscope Zeiss Axioskop 2 in combination with a 20x (Zeiss Plan-Apochromat, air immersion, NA = 0.8), a 40x (Zeiss Plan-Apochromat, oil immersion [see above], NA = 1.3) and a 63x (Zeiss Plan-Apochromat, oil immersion [see above], NA = 1.4) objective, the digital camera Zeiss AxioCam HRc and the software Zeiss AxioVision AC 4.5 (Carl Zeiss Microscopy GmbH). The final micrographs were adjusted for contrast, brightness and tonal values as mentioned above.
Spectral analyses of the resilin autofluorescence
Live female Schistocerca gregaria were frozen at −70°C. For the preparation the locusts were defrosted. The prealar arms and wing hinges were separated using a stereomicroscope (Wild M8, Leica Microsystems GmbH), fine forceps and scissors and a razor blade. The emission spectra of the resilin autofluorescences within eight fresh gnathobases, twelve fresh prealar arms and ten fresh wing hinges were analysed using the confocal laser scanning microscope Leica TCS SP2 equipped with the inverted microscope Leica DM IRBE (Leica Microsystems GmbH). All specimens were embedded in glycerine and mounted on object slides as described above. Because an inverted microscope had to be used, liquid glycerine jelly (Merck KGaA, Darmstadt, Germany) with a temperature of 40°C was placed around the reinforcement rings in order to fix the cover slips to the object slides. Subsequently, the preparations were stored in a fridge at 4°C for 5 h so that the glycerine jelly was hardened. The microscope was equipped with a UV laser (Ar UV, 50 mW, 351 nm and 364 nm), which was used for the analyses. As the system did not contain an acousto-optical tunable filter, both UV laser lines had to be applied simultaneously. A 10x (Leica HC PL APO 10x/0.40 IMM CS; prealar arms and wing hinges) and a 40x (Leica HCX PL APO 40x/1.25-0.75 OIL CS; gnathobases) objective were used, both with immersion oil from Leica Microsystems GmbH (refractive index = 1.518). Optical sections (pinhole size = 1 Airy unit) through the structures of interest were visualized with an image size of 1024×1024 pixels and a frame average of 4. The scan speed was set to 400 Hz. Laser power, detector gain and detector offset were adjusted so that 1) the photobleaching of the autofluorescence was negligible and 2) the intensity of the autofluorescence signals obtained from the structures of interest at the emission maximum was maximal with simultaneous prevention of oversaturation. The emission range from 370 nm to 680 nm was analysed by detecting emission signals from 50 overlapping sections with a bandwidth of 10 nm each.
References
Longhurst, A. R. The structure and evolution of plankton communities. Prog. Oceanogr. 15, 1–35 (1985).
Verity, P. G. & Smetacek, V. Organism life cycles, predation and the structure of marine pelagic ecosystems. Mar. Ecol. Prog. Ser. 130, 277–293 (1996).
Koehl, M. A. R. & Strickler, J. R. Copepod feeding currents: food capture at low Reynolds number. Limnol. Oceanogr. 26(6), 1062–1073 (1981).
Paffenhöfer, G.-A., Strickler, J. R. & Alcaraz, M. Suspension-feeding by herbivorous calanoid copepods: a cinematographic study. Mar. Biol. 67, 193–199 (1982).
Strickler, J. R. Calanoid copepods, feeding currents and the role of gravity. Science 218, 158–160 (1982).
Price, H. J., Paffenhöfer, G.-A. & Strickler, J. R. Modes of cell capture in calanoid copepods. Limnol. Oceanogr. 28(1), 116–123 (1983).
Itoh, K. A consideration on feeding habits of planktonic copepods in relation to the structure of their oral parts. Bull. Plankton Soc. Japan 17, 1–10 (1970).
Michels, J. & Schnack-Schiel, S. B. Feeding in dominant Antarctic copepods – does the morphology of the mandibular gnathobases relate to diet? Mar. Biol. 146, 483–495 (2005).
Nishida, S. & Ohtsuka, S. Specialized feeding mechanism in the pelagic copepod genus Heterorhabdus (Calanoida: Heterorhabdidae), with special reference to the mandibular tooth and labral glands. Mar. Biol. 126, 619–632 (1996).
Smetacek, V. Diatoms and the ocean carbon cycle. Protist 150, 25–32 (1999).
Hamm, C. E. et al. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421, 841–843 (2003).
Beklemishev, K. V. The discovery of silicious formations in the epidermis of lower crustacea (in Russian). Dolk. Akad. Nauk. SSSR 97, 543–545 (1954); English translation by McLean, C. A.; Trans. 29, Ministry of Agriculture, Fisheries and Food.
Sullivan, B. K., Miller, C. B., Peterson, W. T. & Soeldner, A. H. A scanning electron microscope study of the mandibular morphology of boreal copepods. Mar. Biol. 30, 175–182 (1975).
Miller, C. B., Nelson, D. M., Weiss, C. & Soeldner, A. H. Morphogenesis of opal teeth in calanoid copepods. Mar. Biol. 106, 91–101 (1990).
Bhushan, B. Modern Tribology Handbook, Volume Two (CRC Press, Boca Raton, 2000).
Michels, J. & Gorb, S. N. Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J. Microsc. 245(1), 1–16 (2012).
Durbin, E. & Kane, J. Seasonal and spatial dynamics of Centropages typicus and C. hamatus in the western North Atlantic. Prog. Oceanogr. 72, 249–258 (2007).
Martynova, D. M., Graeve, M. & Bathmann, U. V. Adaptation strategies of copepods (superfamily Centropagoidea) in the White Sea (66°N). Polar Biol. 32, 133–146 (2009).
Greve, W., Reiners, F., Nast, J. & Hoffmann, S. Helgoland Roads meso- and macrozooplankton time-series 1974 to 2004: lessons from 30 years of single spot, high frequency sampling at the only off-shore island of the North Sea. Helgol. Mar. Res. 58, 274–288 (2004).
Krause, M., Dippner, J. W. & Beil, J. A review of hydrographic controls on the distribution of zooplankton biomass and species in the North Sea with particular reference to a survey conducted in January-March 1987. Prog. Oceanogr. 35, 81–152 (1995).
Andersen, S. O. & Weis-Fogh, T. Resilin. A rubberlike protein in arthropod cuticle. Adv. Insect Physiol. 2, 1–65 (1964).
Anraku, M. & Omori, M. Preliminary survey of the relationship between the feeding habit and the structure of the mouth-parts of marine copepods. Limnol. Oceanogr. 8(1), 116–126 (1963).
Conley, W. J. & Turner, J. T. Omnivory by the coastal marine copepods Centropages hamatus and Labidocera aestiva. Mar. Ecol. Prog. Ser. 21, 113–120 (1985).
Saage, A., Vadstein, O. & Sommer, U. Feeding behaviour of adult Centropages hamatus (Copepoda, Calanoida): Functional response and selective feeding experiments. J. Sea Res. 62(1), 16–21 (2009).
Antajan, E. Responses of calanoid copepods to changes in phytoplankton dominance in the diatom-Phaeocystis globosa dominated Belgium coastal waters. PhD thesis. Vrije Universiteit Brussel. 147 pp (2004).
Fowler, S. W. & Fisher, N. S. Viability of marine phytoplankton in zooplankton fecal pellets. Deep-Sea Res. 30, 963–969 (1983).
Gibson, L. J. & Ashby, M. F. Cellular Solids: Structure and Properties (Pergamon Press, New York, 1988).
Wang, R. Z. & Weiner, S. Strain-structure relations in human teeth using Moiré fringes. J. Biomech. 31(2), 135–141 (1998).
Weis-Fogh, T. Molecular interpretation of the elasticity of resilin, a rubber-like protein. J. Mol. Biol. 3, 648–667 (1961).
Fong, H., Sarikaya, M., White, S. N. & Snead, M. L. Nano-mechanical properties profiles across dentin-enamel junction of human incisor teeth. Mat. Sci. Eng. C-Bio. S. 7, 119–128 (2000).
Zheng, J., Zhou, Z. R., Zhang, J., Li, H. & Yu, H. Y. On the friction and wear behaviour of human tooth enamel and dentin. Wear 255, 967–974 (2003).
Andersen, S. O. Characterization of a new type of cross-linkage in resilin, a rubber-like protein. Biochim. Biophys. Acta 69, 249–262 (1963).
Weis-Fogh, T. A rubber-like protein in insect cuticle. J. Exp. Biol. 37, 889–907 (1960).
Neville, A. C. Growth and deposition of resilin and chitin in locust rubber-like cuticle. J. Insect Physiol. 9, 265–278 (1963).
Smetacek, V. A watery arms race. Nature 411, 745 (2001).
Ianora, A., Poulet, S. A. & Miralto, A. The effects of diatoms on copepod reproduction: a review. Phycologia 42(4), 351–363 (2003).
Ianora, A. & Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review. Ecotoxicology 19, 493–511 (2010).
Lochte, K., Ducklow, H. W., Fasham, M. J. R. & Stienen, C. Plankton succession and carbon cycling at 47°N 20°W during the JGOFS North Atlantic Bloom Experiment. Deep-Sea Res. II 40(1/2), 91–114 (1993).
Grattepanche, J.-D., Breton, E., Brylinski, J.-M., Lecuyer, E. & Christaki, U. Succession of primary producers and micrograzers in a coastal ecosystem dominated by Phaeocystis globosa blooms. J. Plankton Res. 33(1), 37–50 (2011).
Pannard, A., Claquin, P., Klein, C., Le Roy, B. & Véron, B. Short-term variability of the phytoplankton community in coastal ecosystem in response to physical and chemical conditions' changes. Estuar. Coast Shelf Sci. 80, 212–224 (2008).
Alvain, S., Moulin, C., Dandonneau, Y. & Loisel, H. Seasonal distribution and succession of dominant phytoplankton groups in the global ocean: A satellite view. Global Biogeochem. Cy. 22, GB3001; DOI:10.1029/2007GB003154 (2008).
Bennet-Clark, H. C. & Lucey, E. C. A. The jump of the flea: a study of the energetics and a model of the mechanism. J. Exp. Biol. 47, 59–76 (1967).
Young, D. & Bennet-Clark, H. C. The role of the tymbal in cicada sound production. J. Exp. Biol. 198, 1001–1019 (1995).
Gorb, S. N. Serial elastic elements in the damselfly wing: mobile vein joints contain resilin. Naturwissenschaften 86, 552–555 (1999).
Wiltshire, K. H. et al. Helgoland Roads, North Sea: 45 Years of Change. Estuar. Coast. 33, 295–310 (2010).
Bechstein, K., Michels, J., Vogt, J., Schwartze, G. C. & Vogt, C. Position-resolved determination of trace elements in mandibular gnathobases of the Antarctic copepod Calanoides acutus using a multimethod approach. Anal. Bioanal. Chem. 399, 501–508 (2011).
Michels, J. & Büntzow, M. Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. J. Microsc. 238(2), 95–101 (2010).
Hodson, M. J., Smith, R. J., van Blaaderen, A., Crafton, T. & O'Neill, C. H. Detecting plant silica fibres in animal tissue by confocal fluorescence microscopy. Ann. Occup. Hyg. 38, 149–160 (1994).
Acknowledgements
This project was part of the virtual institute ‘PlanktonTech’, which was funded by the Helmholtz Society. Leona Schulze, Anna Bertram and Lars Friedrichs helped with the processing of the zooplankton samples. The Biologische Anstalt Helgoland (Alfred Wegener Institute for Polar and Marine Research) and in particular Margret Krüß, Petra Müller and Markus Molis kindly helped to get zooplankton samples and provided lab space and facilities. The sampling of zooplankton by the crew of the research motorboat ‘Aade’ is gratefully acknowledged. Ulf Bickmeyer (Alfred Wegener Institute for Polar and Marine Research, Bremerhaven) provided access to the Leica TCS SP2. Anneke Michels made the linguistic revision of the manuscript. The comments and suggestions of three anonymous referees are strongly appreciated.
Author information
Authors and Affiliations
Contributions
J.M. designed the research and performed the major part of it. J.V. conducted the µ-PIXE measurements. J.M. and S.N.G. analysed the data. J.M. wrote the manuscript. S.N.G. and J.V. contributed to the writing of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Michels, J., Vogt, J. & Gorb, S. Tools for crushing diatoms – opal teeth in copepods feature a rubber-like bearing composed of resilin. Sci Rep 2, 465 (2012). https://doi.org/10.1038/srep00465
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00465
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.