Abstract
The phase behaviour of a system composed of amino acid-based surfactant (sodium N-lauroylsarcosinate hydrate), 1-decanol and deionised water was investigated for vesicle formation. Changing the molar ratio of the amphiphiles, two important aggregate structures were observed in the aqueous corner of the phase diagram. Two different sizes of microemulsions were found at two amphiphile-water boundaries. A stable single vesicle lobe was found for 1∶2 molar ratios in 92 wt% water with vesicles approximately 100 nm in size and with high zeta potential value. Structural variation arises due to the reduction of electrostatic repulsions among the ionic headgroups of the surfactants and the hydration forces due to adsorbed water onto monolayer's. The balance of these two forces determines the aggregate structures. Analysis was followed by the molecular geometrical structure. These findings may have implications for the development of drug delivery systems for cancer treatments, as well as cosmetic and food formulations.
Similar content being viewed by others
Introduction
Studies of vesicle formation and morphology for potential applications are rapidly growing areas of research1. The research acquires immense importance owing to the ability of vesicles to mimic cell properties. A vesicle acts as a nanocarrier with its capacity to encapsulate water-soluble drugs in its aqueous core and water-insoluble drugs in its membrane2,3. Beyond phospholipids, a number of synthetic surfactants are known to self-assemble into vesicle structures4,5,6,7,8. These surfactants exhibit a wide variety of self-assembled structures, all of which have potential applications in drug delivery and, cosmetic and food formulations. However, the toxic effects of many synthetic surfactants have limited their use in these fields. This adverse consequence can be avoided by selecting a suitable biocompatible system for vesicle preparation.
In recent years, there has been an increasing interest in using biosurfactants because of their numerous advantages including biocompatibility, biodegradability and easy production from renewable natural sources8,9,10,11,12,13. Despite their potential advantages, vesicle formation from amino acid-based biosurfactants is limited. In this study, we used one single chain amino acid-based surfactant, sodium N-lauroylsarcosinate hydrate (SNLS), which possesses all of these potential advantages. Due to a bulky ionic polar head and strong electrostatic repulsion, the single chain ionic surfactant always forms micelles in pure water14. Furthermore, like other fatty acid vesicles, it forms bilayers or vesicles in the presence of other neutral amphiphiles, which doubles the hydrocarbon volume15,16,17. An ion pair formed by the association of two headgroups of ionic and neutral surfactants induces a bilayer structure.
Many have reported that the addition of alcohol (particularly long chain alcohols) favours vesicle formation18,19,20,21,22,23. Andega et al.24 demonstrated in their study that the permeation-enhancing property through porcine and human skin increases with the alcohol chain length up to 1-decanol and then decreases again with longer chains. Several other investigations regarding the permeation-enhancing property of alcohols in the vesicles25,26 and bilayers27 have been reported. Any primary alcohol with a C10 to C20 chain is a good and biodegradable surfactant28. Based on their toxicological studies, Nelson et al.29 reported that 1-decanol is fully nontoxic even for the foetal rats. Considering these facts, we used 1-decanol as a cosurfactant for vesicle formation.
Generally, a mixture of water-soluble and water-insoluble surfactants results in vesicle formation after immersion in water30. With SNLS and 1-decanol, vesicles are formed when the mixture of the two amphiphiles (at a particular molar ratio) is immersed into water. In fact, the displacement of the adsorbed nonpolar surfactant and their insertion into the hydrophobic area of the monolayer are the driving forces of this process. Packing of the acyl chains of 1-decanol with that of the surfactants increases the hydrophobic area. At the same time, the presence of an OH group between two amino groups forcefully reduced the head group area. The duel effects are responsible for vesicle formation.
The purpose of this study was to develop a non-toxic and low cost drug delivery system. The detailed phase behaviour was studied in the water-rich corner. Rich morphology was observed from the surfactant- rich area to the 1-decanol rich area. The 1∶2 molar ratio of surfactant/1-decanol at 92 wt% water is the most stable point based on macroscopic observation for six months. Prepared vesicles are stable at comparatively higher amphiphile concentrations. The long term stability of this vesicle-forming system will make it promising for drug delivery.
Results
Before examining the use of 1-decanol for vesicle formation, we attempted vesicle formation with ethanol, 1-heptanol, 1-hexanol and 1-octanol as cosurfactants. The ethanol solution was clear and non-birefringent between cross polarisers, which is indicative of the formation of mixed micelles. However, only lamella was formed after treatment with 1-heptanol, 1-hexanol and 1-octanol. The formation of lamella was confirmed with the observation of their rainbow texture through cross polarisers. In contrast, we obtained vesicles for a wide range of molar ratios with 1-decanol. Between cross polarisers, floating birefringent was observed. In addition, transmission electron microscopy (TEM) and dynamic light scattering (DLS) also indicated the presence of vesicles in solution. These results imply that the chain lengths of the cosurfactant influence vesicle formation.
Phase behaviour
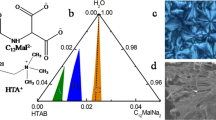
We have examined the phase behaviour of the water-rich region of the system. Based on our investigations, we have drawn a phase diagram of the water-rich corner (see Fig. 1 (a)). With 0.04 wt% 1-decanol, the surfactant formed a slightly bluish turbid phase consisting of a small viscous cloudy upper phase (2–5%). Furthermore, this upper phase increased with the increasing concentration of both amphiphiles. The samples remained in two phases up to 96 wt% water. Two isotropic single phases were found at each amphiphile-water border. The first isotropic phase was situated in the border of the surfactant-water region (SNLS/1-decanol ≥ 8∶5, denoted as “I”). This phase was clear but scattered some light, while the other phase was isotropic and milky white and appeared at the 1-decanol-water border (SNLS/1-decanol ≥ 1∶8.5 termed as “V”). A single vesicle lobe was found in the intermediate region (between the molar ratios of 5∶6.5 and 1∶4 in 95–90 wt% water) of the phase diagram. This region is termed as ‘III’ in the phase diagram. A greater portion of the phase diagram was two phase. For molar ratios of 8∶5–5∶6.5 (termed as II) and 1∶4–1∶8.5 (region IV) of SNLS/1-decanol, the solution remained two phase. The macroscopic appearance of the lower phase changed from clear to milky white with increasing concentrations of 1-decanol. In both isotropic phases, microemulsion was the dominant structure, differing in the size range of the particles. In the surfactant-rich side, the size of the particles was approximately 35 nm, whereas in the decanol-rich side, their size was found approximately 300 nm.
The phase diagram of sodium N-lauroylsarcosinate hydrate/1-decanol/water systems at 25°C. The shaded area represents a two phase region.
The samples' macroscopic appearance is shown with their corresponding TEM images (followed by a negative staining technique), with, (a) a phase diagram of the system; (b) and (c) the TEM image of the upper and lower phase of the sample at 97 wt% water in equimolar ratio of SNLS and 1-decanol; (d) TEM image of the microemulsion for the molar ratio of 1∶9.3 in 94 wt% water; and (e) TEM image of vesicles for the molar ratio of 1∶2 in 92 wt% water.
Morphology of the self-assembled structure
The observed microstructures were characterised by TEM (see Fig. 1). In region ‘III’ of the phase diagram, the most bluish phase was found at a molar ratio of 1∶2 in 92 wt% water. The TEM image of the obtained vesicles in this region is shown in Figure 1(e). The size of these particles was found approximately 100 nm, which is also comparable with the result obtained from the DLS analysis (Fig. 2). The TEM image clearly shows the internal cavity surrounded by a brighter border. Figures 1(b) and 1(c) represent one vesicle and microemulsion phase at 97 wt% water at a molar ratio of 1∶1 SNLS/1-decanol. The bottom phase contains a microemulsion of approximately 40 nm and the top phase contains vesicles approximately 200 nm in diameter. The trend of increasing vesicle size may be due to the reduction of electrostatic repulsion between inter-vesicular dispersion. This result indicates that the proper mole fraction of amphiphiles is necessary; otherwise, the sample is rendered into two phases. Figure 1(d) represents one microemulsion phase of approximately 300 nm in diameter. It is clear from the phase map that microemulsion is the dominant structure above the equimolar ratio and on the 1-decanol rich side of the phase diagram.
From the small angle X-ray scattering investigation of the vesicle dispersion (SNLS/1-decanol molar ratio = 1∶2 in 92 wt% water), it was found that the scattering spectra consists of two peaks, a broad scattering peak and a small sharp peak (Fig. 3). The broad peak represents a single bilayer31,32 and the second sharp peak arises from the interparticle interference33. For the confirmation of the vesicle morphology in dry conditions, we dried the sample in a desiccator. The broad scattering peak of the dry vesicles appeared at the same position in both cases, indicating that the vesicle morphology did not change even in dry conditions. The second peak changed according to the concentration of vesicles. The reason behind the observed stability may be due to the high absolute value of the zeta potential (42 mV, see Fig. 4). This strong repulsive force prevents the agglomeration of vesicles. Considering all of the experimental results, we selected the molar ratio of 1∶2 at 92 wt% water as the most stable point in the system.
Figure 5 shows the FTIR spectra of the vesicle solution. Maxima were found at 1656 cm−1 (amide region), which is shifted from a split peak in the surfactant at 1640 and 1648 cm−1. The shift is attributed to the hydrogen bonding between the C = O of the amide group and the alcoholic hydroxyl group. Tenaciously bound water also contributes to this bonding. Strong hydrogen bonding yields a significant band broadening in region of 3400 cm−1. Both OH stretching vibrations from the alcohol group and adsorbed water contributes this broadening. However, a hydrogen band at 3304–3229 cm−1 is an evidence of the tertiary ammonium cation (R3N+H)34. In the case of the micelle solution, a single very broad peak was observed at 3400 cm−1, while no peak was observed at 3304–3229 cm−1. Strong hydrogen bonding between water and the amino acid head group is responsible for such a peak in the micelle solution. In the vesicle solution, a reduction of hydrogen bonding with water occurs. The most probable reason for such an occurrence is the tendency towards a reduction of interfacial hydration due to the addition of 1-decanol. The strong asymmetric and symmetric stretch of methyl group of surfactant and 1-decanol appears as very weak stretching vibration at the peak value 2956 cm−1 and 2856 cm−1 in vesicle solution, because the association of more acyl chains weakens the peak strength.
Discussion
The phase map shows significant changes of the self-assembled properties. In both surfactant-rich and decanol-rich regions (region I and V) of the phase map, the presence of excess ionic surfactant accumulates around some free 1-decanol molecules. Consequently, the hydrophilic surface of each monolayer remains in contact with water and the hydrophobic surface remains in contact with excess 1-decanol. The interfacial tension is smaller in the region of water and the hydrophilic surface monolayer than that of the region of 1-decanol and the hydrophobic surface. As a result, the hydrophilic surface swells and the hydrophobic surface contracts. Thus, the convexing of the surfactant monolayer with respect to the water phase containing the oil phase (1-decanol) in its interior, forms a micro emulsion. The size of the aggregates depends on the relative concentration of the hydrophilic and hydrophobic areas. Specifically, a larger hydrophilic area favours the formation of a microemulsion of below 40 nm in size in the surfactant-rich corner. However, at the same water concentration, a comparatively large hydrophobic area favours the formation of a larger microemulsion. The size of the particles depends on the availability of 1-decanol molecules. In the intermediate region, i.e., area III in the phase diagram, the situation is better for a bilayer structure.
For vesicle formation, two major phases must be fulfilled in a system. One is the requirement of energy; i.e., the energy level of vesicle phase, should be lower than that in molecular dispersion state19. The other requirement is the molecular packing parameter introduced by Israelachvili35. In fact, the packing of the acyl chain in the hydrophobic core determines the aggregate structure and is defined as the ratio of the volume of the hydrocarbon tail of the surfactant in the core (vc) and the product of the optimal head group area (a0) and the critical chain length of the tail (lc).

The spherical micelles are formed at 0<P<1/3 and the cylindrical micelles are formed at 1/3<P<1/2. For 1/2<P<1, vesicles are formed, at P≈1, lamella formed and for P>1, reverse micelles are formed.
It is clear that, a certain concentration of 1-decanol must be used for vesicle formation in this system. Addition of decanol at a molar ratio of 1∶2 shows the most bluish and turbid phase in this system, which implies that the insertion of decanol molecules at 92 wt% water enlarges the hydrophobic area, which is beneficial for vesicle or bilayer formation. Similar results were also found in other systems. It was previously shown that a specific molar ratio is beneficial for vesicle formation15 and the importance of the interfacial properties of a mixed surfactant system36 were also emphasised.
Generally, in a system consisting of an ionic surfactant and a cosurfactant, the system balanced at some optimum mixing ratio37. Regarding this matter, it can be assumed that, the maximum solubilisation of amphiphiles is achieved in the intermediate region (region III) of the phase diagram. We considered this region to be the solubilisation boundary. In catanionic systems, both cationic-rich38 anionic-rich39 vesicle lobe regions were reported, but precipitates at equimolar ratios, i.e., excess surfactant, should be present within these bilayers. Zhai et al.40 also showed that an aqueous anionic and neutral amphiphile mixture forms vesicles at certain molar ratios. The excess amphiphiles weaken the repulsive forces between two ionic head groups. The combination of reduced interfacial hydration, as well as weak repulsion, makes the polar head group area smaller than the micelle and the packing of acyl chain increases the hydrophobic area. The combination of these effects reduces the free energy of the system and also favours the formation of a cup-like structure, which is the basis for vesicle formation.
With the increase in bonding interactions, the two molecules closer together, were making more space to for new 1-decanol molecules to enter. Due to the strong hydrogen bonding property, the new OH group also associates with adsorbed water and with other surrounding headgroups. It is notable that not all mixing ratios yielded vesicle formation, however, a molar ratio of 5∶6.5–1∶4 (SNLS/1-decanol) in 95–90 wt% water is sufficient for vesicle formation. Adequate insertion of 1-decanol leads to the decrease of the curvature, consequently closing the bilayer, which then contains a fraction of the aqueous part in its core. Furthermore, the compressed bilayers and adsorbed water on the monolayer prevents the outer molecules from entering the inner layer of the vesicle. This effect limits the diameter of the vesicles to a certain range.
In conclusion, the sodium N-lauroylsarcosinate hydrate/ 1-decanol/water system has similar properties of forming bilayer structures to that of an oppositely-charged amphiphile system. Furthermore, different aggregate structures can be achieved by changing the mixing ratio of both amphiphiles. Previously, it was reported that the addition of alcohol is beneficial for vesicle formation in some mixed surfactant systems. However, 1-decanol enhances not only the vesicle formation, but also two different sizes of microemulsion phases in one single chain ionic surfactant. Vesicles were stable between the temperatures of 20 and 37°C. This result indicates that, changes in interfacial hydration and the interactions between amphiphiles in different mixing ratios greatly change the phase behaviour. Additionally, a similar chain length (C11 for the surfactant and C10 for the 1-decanol) greatly influences the hydrophilic and hydrophobic portion. Due to these properties, the system is promising for development into a nanocarrier-forming system.
Methods
Materials and sample preparation
Sodium N-lauroylsarcosinate hydrate (SNLS) was purchased from TCI (Japan), 1-decanol with 97% purity was purchased from Fluka-Chemica (Switzerland). Deionised water was used to prepare all of the samples. To obtain vesicles, the desired amounts of amphiphile were measured in the glass tube and then the required amount of water was added. The resulting suspension was sonicated at 20°C for 10 min using a bath-type sonicator. To obtain microemulsion, the mixture was vortexed for 5 min. The homogenous dispersions were then centrifuged at 4,000 rpm for 10 min to see the phase separation. Before analysis, all samples were left undisturbed at equilibration for several days.
Phase diagram determination
After equilibration, inspection between cross polarisers was performed to identify the birefringent and isotropic phase. We studied the expected phase sequence over the composition range 0.08–6 wt% of surfactant and 1-decanol and 90–99.92 wt% water. The composition of each solution was expressed in molar fraction (αs) and defined in equation (1)

where, [surfactant] and [1-decanol] are the molar concentrations of surfactant and 1-decanol.The macroscopic appearance was monitored at regular intervals by both visual inspection and between cross polarisers after subsequent centrifugation.
It was observed that the samples' appearance remained unchanged in the temperature range of 20–35°C. The macroscopic appearance of samples is shown in Figure 1 with their corresponding TEM images. This inspection was conducted over the course of months to check the phase separation or flocculation above the isotropic or vesicular region.
Transmission electron microscopy (TEM)
Observation of the vesicle formation was examined using TEM (Philips-CM12). A drop of vesicle dispersion was applied to the carbon grid and left for partial drying to allow some of the vesicles to adhere to the carbon grid. The excess sample was removed by filter paper from the opposite direction. A drop of 3% uranyl acetate solution was added to the grid and left for 10 sec. Again, the excess solution was removed washing the grid thrice. Each time the liquid was adsorbed with filter paper and the sample was dried in the air. The sample was then characterised under an electron microscope at an accelerating voltage of 100 kV.
Measurement of the particles
Vesicle size and zeta potential were determined with a Zetasizer Nano ZS (Malvern Instruments, Herrenberg, Germany) after equilibration. Z-average particle sizes were measured at the scattering angle of 90° at 25°C using DLS combined with Malvern's DTS software (v.5.02). Zeta potential values were measured by laser Doppler anemometry at 25°C and calculated using DTS software.
Further investigation of the vesicular phase was performed by small angle scattering X-ray technique at 25°C using a kratky compact small angle system, which is equipped with a position-sensitive detector containing 1024 channels of 54 mm in width. The wavelength used was 0.154 nm and the sample-detector distance was 274 mm. A few milligrams of sample were enclosed in the sample holder on a flat miler sheet. Data were normalised for an acquisition time for 30 min. The results are represented in Figure 3 after background subtraction.
The Fourier transform infrared (FTIR) spectra were recorded using a Perkin Elmer Spectrum BX FTIR system over the region of 4000–400 cm−1 of the sample solution. Prior to the assay, liquid samples were prepared by placing a few drops of the vesicle solution between two sodium chloride (NaCl) salt plates.
References
Lovell, J. F. et al. Porphysome nanovasicles generated by porphyrin bilayers for use as multimodal biphotonic contrast agents. Nature Mater. 10, 324–332 (2011).
Chu, C. et al. Proliposomes for oral devivery of dehydrosilymarin: preparation and evaluation in vitro and in vivo . Acta Pharmacol.Sin. 32, 973–980 (2011).
Yang, T. et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int. J. Pharm. 338, 317–326 (2007).
Fendler, J. H. Membrane Mimetic Chemistry (Willey, New York, 1982).
Jones, M. N. The surface properties of phospholipid liposome systems and their characterization. Adv. Colloid Interface Sci. 54, 93–128 (1995).
Makino, K. & Shibata, A. Surface properties of liposomes depending on their composition. in Advances in Planar Lipid Bilayers and Liposomes (ed. Liu A. L.) 4, 49–53 (Elsevier, 2006).
Bandyopadhyay, P. & Neeta, N. S. Evidence of vesicle formation from 1∶1 nonionic surfactant span 60 and fatty alcohol mixtures in aqueous ethanol: Potential delivery vehicle composition. Colloids Surf. B 58, 305–308 (2007).
Rosa, M., Moran, M., Miguel, M. & Lindman, B. The association of DNA and stable catanionic amino acid-based vesicles. Colloids Surf. A 301, 361–375 (2007).
Mulligan, C. N. Recent advances in the environment applications of biosurfactants. Curr. Opi. Colloid Interface Sci. 14, 372–378 (2009).
Ruiz, C. C., Hierrezuelo, J. M. & Molina-Bolívar, J. A. Effect of glycine on the surface activity and micellar properties of N-decanoyl-N-methylglucamide. Colloid Polym. Sci. 286, 1281–1289 (2008).
Muthusamy, K., Gopalakrishnan, S., Ravi, T. K. & Sivachidambaram, P. Biosurfactants: Properties, commercial production and application.Curr. Sci. 94, 736–747 (2008).
Worakitkanchanakul, W. et al. Aqueous phase behavior and vesicle formation of natural glycolipid biosurfactants, mannosylerythritol lipid-B. Colloids Surf. B 68, 207–212 (2009).
Ohta, A., Toda, K., Morimoto, Y., Asakawa, T. & Miyagishi, S. Effect of the side chain of N-acyl amino acid surfactants on micelle formation: An isothermal titration calorimetry study. Colloids Surf. A 317, 316–322 (2008).
Zemb, T., Dubois, M., Deme, B. & Gulik-Krzywicki, T. Self-Assembly of flat nanodiscs in salt-free catanionic surfactant solutions. Science 283, 816–819 (1999).
Gradzielski, M., Müller, M., Bergmeier, M., Hoffmann, H. & Hoinkis, E. Structural and macroscopic characterization of a gel phase of densely packed monodisperse, unilamellar vesicles. J. Phys. Chem. B 103, 1416–1424 (1999).
Morigaki, K. & Walde, P. Fatty acid vesicles. Curr. Opi. Colloid Interface Sci. 12, 75–80 (2007).
Vlachy, N. et al. Spontaneous formation of bilayers and vesicles in mixtures of single-chain alkyl carboxylates: effect of pH and aging and cytotoxicity studies. Langmuir 24, 9983–9988 (2008).
Seo, D. et al. Two-dimensional packing patterns of amino acid surfactant and higher alcohols in an aqueous phase and their associated packing parameters. J. Colloid Interface Sci. 273, 596–603 (2004).
Huang, J. B., Zhu, B. Y., Zhao, G. X. & Zhang, Z. Y. Vesicle formation of a 1∶1 catanionic surfactant mixture in ethanol solution. Langmuir 13, 5759–5761 (1997).
Huang, J., Zhu, Y., Zhu, B., Li, R. & Fu, H. Spontaneous vesicle formation in aqueous mixtures of cationic surfactants and partially hydrolyzed polyacrylamide. J. Colloid Interface Sci. 236, 201–207 (2001).
Hoffmann, H., Thunig, C. & Miller, D. Vesicle phases from N-methyl-N-alkanoylglucamin and various co-surfactants. Colloids Surf. A 210, 147–158 (2002).
Danino, D. et al. Microstructures in the aqueous solutions of a hybrid anionic fluorocarbon/hydrocarbon surfactant. J. Colloid Interface Sci. 259, 382–390 (2003).
Gradzielski, M., Bergmeier, M., Hoffmann, H., Mueller, M. & Grillo, I. Vesicle gel formed by a self-organization process. J. Phys. Chem. B 104, 11594–11597 (2000).
Andega, S., Kanikkanan, N. & Singh, M. Comparison of the effect of fatty alcohols on the permeation of melatonin between porcine and human skin. J. Controlled Release 77, 17–25 (2001).
Oliveira, G., Beezer, A. E., Hadgraft, J. & Lane, M. E. Alcohol enhanced permeation in model membranes. Part I. Thermodynamic and kinetic analyses of membrane permeation. Int. J. Pharm. 393, 61–67 (2010).
Williams, A. C. & Berry, B. W. Penetration enhancers. Adv. Drug Deliv. Rev. 56, 603–618 (2004).
Greipernau, B., Leis, S., Schneider, F., Sikor, M., Steppich, D. & Bockmann, R. A. 1-Alkanols and membranes: A story of attraction. Biochim. Biophy. Acta 1768, 2899–2913 (2007).
Nelson, R. Dispersing Powders in Liquid. 4, 1–8 (Elsevier 2003).
Nelson, B. K., Brightwell, W. S., Khan, A., Krie, E. Fg. & Hoberman, A. M., Jr Developmental toxicology assessment of 1-octanol, 1-nonanol and 1-decanol administered by inhalation to rats. Int. J. Toxicol. 9, 93–97 (1990).
Lasic, D. D., Kidric, J. & Zagorc, S. A simple method for the preparation of small unilameller vesicles. Biochim. Biophys. Acta 896, 117–122 (1987).
Hauser, H. Naturally occurring amphiphiles: aspects of their phase behavior. in Reverse Micelles (eds. Luisi P. L. & Straub B. E.) 37–53 (Plenum Press, New York, 1984).
Riske, K. A., Amaral, L. Q., Döbereiner, H. -G. & Lamy, M. T. Mesoscopic structure in the chain-melting regime of anionic phospholipid vesicles: DMPG. Biophy. J. 86, 3722–3733 (2004).
Feigin, L. A. & Svergun, D. I. Structure Analysis by Small-Angle X-Ray and Neutron Scattering (ed. Taylor G. W.) (Plenum Press, New York, 1987).
Hummel, D. O., Baum, A., Liu, M., Pastura, A. & Weber, A. Analysis of surfactants: Atlas of FTIR-spectra with interpretations (Hanser Gardner Publications, Germany, 1996).
Israelachvili, J. N. Intermolecular and Surface Forces (Academic Press, 1992).
Patist, A., Devi, S. & Shah, D. O. Importance of 1∶3 Molecular Ratios on the Interfacial Properties of Mixed Surfactant Systems. Langmuir 15, 7403–7405 (1999).
Gillberg, G., Lehtinen, H. & Friberg, S. NMR and IR investigation of the conditions determining the stability of microemulsions. J. Colloid Interface Sci. 33, 40–53 (1970).
Marques, E. F., Regev, O., Khan, A., Graca Miguel, M. & Lindman, B. Vesicle formation and general phase behaviour in the catanionic mixture SDS-DDAB-water. The anionic rich side. J. Phys. Chem. B 102, 6746–6758 (1998).
Marques, E. F., Regev, O., Khan, A., Graca Miguel, M. & Lindman, B. Vesicle formation and general phase behaviour in the catanionic mixture SDS-DDAB-water. The cationic rich side. J. Phys. Chem. 103, 8353–8363 (1999).
Zhai, L., Li, G. & Sun, Z. Spontaneous vesicle formation in aqueous solution of zwitterionic and anionic surfactant mixture. Colloids Surf. A 190, 275–283 (2001).
Acknowledgements
The authors would like to thank Universiti Kebangsaan Malaysia for the financial support of this research work through the grant numbers UKM-GUP-NBT-08-27-106, UKM-GGPM-NBT-163-2010 and STGTL-007-2010/11.
Author information
Authors and Affiliations
Contributions
N. A. and S. R. designed the research. N. A. drafted the manuscript. N. A., S. R. and F. M. contributed to the experiments. I. A. R. and M. I. H. R. contributed intellectually. All authors discussed the results and contributed to the revision of the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Akter, N., Radiman, S., Mohamed, F. et al. Ternary phase behaviour and vesicle formation of a sodium N-lauroylsarcosinate hydrate/1-decanol/water system. Sci Rep 1, 71 (2011). https://doi.org/10.1038/srep00071
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00071
This article is cited by
-
Laboratory Characterization of Surfactant-Enhanced Air Sparging Effectiveness on VOC-Contaminated Soil with Low Permeability
Water, Air, & Soil Pollution (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.