« Prev Next »
—Sylvia (10 years old), daughter of Werner Arber (as quoted in Konforti, 2000)
Swiss microbiologist Werner Arber was one of the recipients of the 1978 Nobel Prize in Physiology or Medicine, an award he earned for his discovery (with Stuart Linn) of restriction enzymes, otherwise known by his daughter Sylvia as "servants with scissors." Arber discovered restriction enzymes while studying a phenomenon known as host-controlled restriction of bacteriophages (also known at the time as host-controlled modification of bacteriophages). Bacteriophages are viral particles that invade bacteria and replicate their own DNA independently of the bacterial chromosomal DNA. Prior to Arber's work, researchers Salvador Luria and Mary Human had shown that various phages were host specific, with each phage surviving and flourishing only in one host bacterial strain and growing poorly in others (Luria & Human, 1952). Those phages that grew poorly were said to be "restricted" by their host. Arber wanted to know why.
Initial Steps in Restriction Enzyme Research
Arber proposed that bacterial cells (in this case, E. coli) were able to protect themselves against foreign DNA through some sort of enzymatically catalyzed genetic defense mechanism (Arber & Linn, 1969). Specifically, he theorized that only those bacteriophages that had previously been in contact with the same bacterial strain could successfully infect new host cells, and that the previous exposure somehow modified the phage DNA in a way that protected it from restriction. Phages with unmodified DNA, on the other hand, were immediately broken down by enzymes. This occurred because the host cell enzymes recognized these phages as foreign, cleaving their DNA and restricting their growth. Arber further proposed that there were specific sites in the genome at which restriction activities occurred. Arber and Linn referred to the enzyme responsible for this "endonucleolytic scission" as endonuclease R, a name later changed to EcoB. It didn't take long for other scientists to identify a second restriction enzyme in E. coli: EcoK (Meselson & Yuan, 1968).
Soon after the discovery of EcoB and EcoK, microbiologists Hamilton Smith and Kent Wilcox isolated and characterized the first restriction enzyme from a second bacterial species, Haemophilus influenzae. They also confirmed Arber's hypothesis by demonstrating that HindII, the name eventually given to the enzyme they discovered, degrades foreign phage DNA but not the bacterial host's DNA (Smith & Wilcox, 1970). Smith and his postdoctoral fellow, Thomas Kelly, later identified the actual nucleotide sequence of the specific site where HindII cleaves (known today as a restriction or recognition site), again confirming Arber's hypothesis that restriction enzymes are extremely selective with regard to where they make their cuts (Smith & Kelly, 1970).
The Nature of Restriction Enzymes Is More Fully Elaborated
The first three letters of a restriction enzyme's name are abbreviations of the bacterial species from which the enzyme has been isolated (e.g., Eco- for E. coli and Hin- for H. influenzae), and the fourth letter represents the particular bacterial strain. Roman numerals are also used as part of the name when more than one restriction enzyme has been isolated from the same bacterial strain.
Today, scientists recognize three categories of restriction enzymes: type I, which recognize specific DNA sequences but make their cut at seemingly random sites that can be as far as 1,000 base pairs away from the recognition site; type II, which recognize and cut directly within the recognition site; and type III, which recognize specific sequences but make their cut at a different specific location that is usually within about 25 base pairs of the recognition site. EcoB and EcoK are type I enzymes, while HindII and HindIII are type II enzymes.
As originally postulated by Arber, all restriction enzymes serve the purpose of defense against invading viruses. Bacteria protect their DNA by modifying their own recognition sequences, usually by adding methyl (CH3) molecules to nucleotides in the recognition sequences and then relying on the restriction enzymes' capacity to recognize and cleave only unmethylated recognition sequences. Also, as Arber suspected, bacteriophages that have previously replicated in a particular host bacterial strain and survived are similarly modified with methyl-labeled nucleotides and thereby protected from cleavage within that same strain.
Learning to Use Restriction Enzymes
Within just a few years of the initial discoveries of EcoB, EcoK, and HindII, scientists were already testing ways to use restriction enzymes. The first major application was as a tool for cutting DNA into fragments in ways that would make it easier to study and, in particular, identify and characterize genes. A second major use was as a device for recombining, or joining, DNA molecules from different genomes, usually with the goal of identifying and characterizing a gene or studying gene expression and regulation (Heinrichs, 2007).
Cutting with Restriction Enzymes
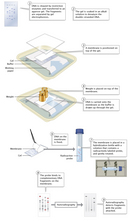
The use of restriction enzymes as a way to cut DNA molecules was first demonstrated in a classic study by Johns Hopkins biochemist Daniel Nathans and his graduate student Kathleen Danna (Danna & Nathans, 1971). This duo used the same experimental methods that Smith and Wilcox had employed to purify HindII from H. influenzae. Nathans and Danna then used the enzyme to cut, or digest, the DNA of the eukaryotic virus SV40 into 11 unique linear fragments. (Found in both monkeys and humans, SV40 has the capacity to cause tumors and was being intensively studied at the time for its cancer-causing potential.) Finally, they separated the fragments using gel electrophoresis, a technique developed in the 1960s and still commonly used as a way to sort nucleic acid molecules of different sizes (Figure 1). "If you look back on it, it seems like such a simple notion," Danna would later recount. "But the fact is, I think [Nathans] saw the utility of this much more clearly than even those of us who were very close to the work. Clearly, he must have had a vision at the very beginning of this that just the simple idea of being able to separate the fragments of viral DNA into specific pieces would have enormous applications" (Brownlee, 2005).
Today, scientists still use restriction enzyme digestion, followed by electrophoresis, as a way to separate DNA fragments. Many scientists also use what is known as a probe, or a DNA or RNA molecule with a base sequence that is complementary to a DNA sequence of interest, to identify where in the genome (i.e., on which fragment) that sequence is located. This basic procedure is outlined in Figure 2. After separating the DNA fragments through electrophoresis, the fragments are transferred from the gel to a solid medium, or membrane. When DNA fragments are separated and transferred in this manner, the process is known as Southern blotting, named after the scientist who developed the technique, Edwin Southern (Southern, 1975). After transfer, the membrane is immersed in a solution of either radioactive or chemically labeled probes. The probes bind to their complementary sequences on the membrane, if any are present. The membrane is then washed, leaving only bound probes that can be detected using autoradiography, if the probes are radioactive, or other means.
Recombining with Restriction Enzymes
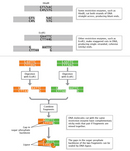
The use of restriction enzymes as a tool for recombining, or joining, different DNA fragments was first illustrated in yet another classic paper, this time by Stanford University researchers Janet Mertz and Ronald Davis (Mertz & Davis, 1972). At the time, scientists had identified the specific site and sequence of cleavage for only one restriction enzyme, HindII. With HindII, cleavage occurred in the middle of a six-base-pair recognition site, yielding what are known as blunt-end fragments (see Figure 3, in which PvuII similarly produces blunt-end fragments). Mertz and Davis discovered that another restriction enzyme, EcoR1, by contrast, cleaves its recognition site in a staggered way that generates fragments with single-stranded overhanging ends known as cohesive, or sticky, ends. In Figure 3, HindIII (not HindII) is used to illustrate how many restriction enzymes make cuts yielding sticky end fragments. After two fragments with complementary sticky ends are joined, the DNA backbone may be covalently sealed using another enzyme called DNA ligase.
Mertz and Davis concluded that because EcoR1 produced sticky end fragments and "all R1 endonuclease-generated ends are identical and complementary, any two DNA molecules with R1 sites can be ‘recombined' at their restriction sites by the sequential action of R1 endonuclease and DNA ligase to generate hybrid DNA molecules." The fact that EcoR1 operates on the same recognition sequence in any piece of DNA from any genome means that it always behaves in the same way, no matter what organism the DNA comes from. This gives molecular biologists powerful tools to create nearly limitless combinations of recombinant DNA. Today, scientists are mixing and matching DNA fragments from different species in ways that continue not only to demonstrate the power of this method, but also to raise serious ethical and social questions.
References and Recommended Reading
Arber, W., & Linn, S. DNA modification and restriction. Annual Review of Biochemistry 38, 467–500 (1969)
Brownlee, C. Danna and Nathans: Restriction enzymes and the boon to molecular biology. Proceedings of the National Academy of Sciences 102, 5909 (2005)
Danna, K., & Nathans, D. Specific changes of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proceedings of the National Academy of Sciences 68, 2913–2917 (1971)
Heinrichs, A. Making the cut: Discovery of restriction enzymes. Nature Milestones. (2007) doi:10.1038/nrg2239 (link to article)
Konforti, B. The servant with the scissors. Nature Structural Biology 7, 99-100 (2000) doi:10.1038/72469 (link to article)
Luria, S. E., & Human, M. L. A nonhereditary, host-induced variation of bacterial viruses. Journal of Bacteriology 64, 557–569 (1952)
Mertz, J. E., & Davis, R. W. Cleavage of DNA by RI restriction endonuclease generates cohesive ends. Proceedings of the National Academy of Sciences 69, 3370–3374 (1972)
Meselson, M., & Yuan, R. DNA restriction enzyme from E. coli. Nature 217, 1110–1114 (1968) doi:10.1038/2171110a0 (link to article)
Smith, H. O., & Kelly, T. J. A restriction enzyme from Hemophilus influenzae. II. Base sequence of the recognition site. Journal of Molecular Biology. 51, 393–409 (1970)
Smith, H. O., & Wilcox, K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. Journal of Molecular Biology 51, 379–391 (1970)
Southern, E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. Journal of Molecular Biology 98, 503–517 (1975).




 Figure 1
Figure 1