« Prev Next »
Metabolic pathways — the chains of chemical reactions that occur in each cell in the body — are affected by a wide range of DNA mutations, including single nucleotide polymorphisms (SNPs); gene deletions, duplications, and insertions; mutations in the regulatory elements of genes; and variations in gene copy number. The identification of gene variants (alleles) that influence drug metabolism is one of the goals of pharmacogenetics, a field of study focused on understanding the genetic basis for differences in drug response among individuals.
The appeal of pharmacogenetics lies in the possibility of personalized medicine. This sort of care has always been the goal of the doctor-patient relationship, with physicians considering a patient's family history and lifestyle when prescribing treatment. Access to information about an individual's genetic makeup would provide yet another source of personalized data and would therefore enable doctors to better define the nature of a disease and find the most effective treatment for a particular patient. With the help of pharmacogenetic studies, physicians will be able to administer treatment regimens that are personalized and adapted to each person's genetic makeup. Accordingly, two people with the same diagnosis might receive different therapies or drug dosages. This might in turn reduce health care costs, because physicians would be able to prescribe more targeted drugs and pharmaceutical companies would be able to develop and market drugs to specific groups of patients.
Codeine is one example of a drug whose metabolism is greatly influenced by genetics. In order for codeine to exert an effect, it first has to be converted into morphine by the body's enzymes. This chemical reaction occurs in the liver and is catalyzed by the cytochrome P450 enzyme, CYP2D6. However, up to 10% of individuals have a mutation in the CYP2D6 gene that abolishes enzyme activity (Wilson et al., 2001; Zanger et al., 2003). Thus, codeine may have very little or no impact on these patients. People with this mutation cannot convert codeine to morphine and thereby cannot benefit from the analgesic effects of the drug. Identification of the CYP2D6 mutation before therapy would allow physicians to prescribe a different pain control regimen, instead of resorting to trial and error.
Finding mutations that affect drug response is not unlike finding mutations that cause disease. Fifty years of clinical studies have repeatedly confirmed that the physiological response to specific chemicals can be inherited (Meyer, 2004). In fact, teasing out the underlying foundations of the genetic variation responsible for these different responses was one of the cornerstones used to build support for the unprecedented effort and expense that was needed to fully sequence the human genome. As researchers look at the entire spectrum of genes and genetic loci that affect an individual's metabolic responses, they are finding that groups of people — often defined according to racial categories — differ in their response to various drugs. However, understanding the basis for these differences has been a challenge.
Race and Pharmacogenetics
Ethnicity and race are both concepts used as a way of categorizing people of shared ancestry and physical traits (Sankar et al., 2007). However, ethnicity also connotes shared cultural, linguistic, behavioral, or religious characteristics. Thus, ethnicity and race are tied to both our genetics and our environment. This complexity makes it much more difficult for researchers to identify the specific connections between pharmacogenetics and race/ethnicity.
In a recent study, Tang et al. (2005) analyzed genetic data from 3,636 people who identified themselves as belonging to one of four major racial and ethnic groups in the United States (Caucasian, African American, East Asian, and Hispanic). Analysis of the data produced four major genetic clusters, which showed near-perfect correspondence with the four self-reported race/ethnicity categories. Tang and colleagues thus concluded that using self-identified race as a way to divide populations in clinical studies is valuable, because that definition encompasses genetic variations.
However, despite the findings of Tang et al., two studies published in the New England Journal of Medicine provide a good example of the problems that can arise when race is used to make conclusions in clinical studies. These two studies focused on treatments for cardiovascular disease (CVD) — a class of diseases that involve the heart or blood vessels; such conditions include high blood pressure, coronary heart disease (heart attack and angina), congestive heart failure, stroke, and congenital heart defects, among others. CVD kills nearly 2,400 Americans each day, and the mortality rate is highest among African Americans. For instance, in 2004, 454 out of every 100,000 African American males died because of CVD, compared to 335 out of every 100,000 Caucasian males, according to the American Heart Association. Similarly, the death rate from CVD was 333.6 per 100,000 for African American females, compared to 238 per 100,000 for Caucasian females. These striking differences in morbidity and mortality, which were apparently linked to race, prompted scientists to ask whether there was a possibility that different standard treatments for CVD had different impacts in different races. Did standard treatments work better in Caucasians than in African Americans?
In 2001, Yancy et al. showed that a drug called carvedilol, which blocks the function of beta-adrenergic receptors, a type of hormone receptor in the body, worked equally well as a treatment for both African American and Caucasian patients with heart failure. However, in a second study (Exner et al., 2001), another drug called enapril, which blocks the action of a protein known as angiotensin-converting enzyme (ACE), was shown to be more effective in Caucasian patients with a particular heart defect than in African Americans. As a result of this study, there was a reported decrease in the offering of ACE inhibitors to treat hypertension in African American patients.
Despite this finding, subsequent studies have shown that when adequate dosing and appropriate combinations are used, ACE inhibitor therapy provides effective blood pressure control in both groups of patients (Saunders & Gavin, 2007). There have also been inconsistencies in results from studies looking at the effects of drugs that block beta-adrenergic receptors (beta blockers). For instance, in 2008, a mutation in G-protein-coupled receptor kinase 5 (GRK5) was found in 40% of African Americans. This mutation protects individuals suffering from heart failure from death and thus has an effect similar to beta-blocker drugs following heart failure (Liggett et al., 2008). The authors concluded that this finding suggests "a reason for conflicting results of beta-blocker clinical trials in this population."
In addition to inconsistent findings among clinical studies that group individuals according to self-defined racial groups, some researchers have voiced fears that such studies could support views that biological differences underlie the social definitions of race. The association of these differences to the social construct of race could potentially usher in a new era of racial discrimination (Sankar et al., 2004). These concerns have prompted scientists to think more broadly about how study design can be improved to find genetic variations related to drug response without dragging social issues of race into the equation.
Redefining Race in Genetic Study Design
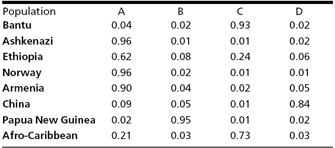
Notably, the data in Table 1 show that 62% of the Ethiopians included in the study were found in the same cluster as most of the Jews, Norwegians, and Armenians, indicating that placement of these individuals in an African American cluster would be at odds with their genetic grouping. Moreover, 21% of Afro-Caribbeans were clustered with the West Eurasians, whereas people from China and New Guinea were almost entirely in separate clusters, revealing that the ethnic label of "Asian" is also an inaccurate description.
Because different populations have different frequencies of various alleles involved in drug metabolism, Wilson et al. next examined variations in genes encoding these enzymes, including CYP2D6, across the clusters they identified with their microsatellite analysis. They found that the genetic groupings (based on the microsatellite analysis) appeared to be more informative regarding differences in drug metabolism than the groupings based on skin color or self-defined ethnic groups.
While the Wilson et al. study supports the premise that understanding an individual's genetic makeup is the key to creating personalized drugs with greater efficacy and safety, it must not be forgotten that environment, diet, age, lifestyle, and state of health all influence a person's response to medicine as well.
References and Recommended Reading
Crawley, L. The paradox of race in the Bidil debate. Journal of the National Medical Association 99, 821–822 (2007)
Evans, W. E., & Relling, M. V. Pharmacogenomics: Translating functional genomics into rational therapeutics. Science 286, 487–491 (1999) doi:10.1126/science.286.5439.487
Exner, D. V., et al. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. New England Journal of Medicine 344, 1351–1357 (2001)
Gillum, R. F. Pathophysiology of hypertension in blacks and whites: A review of the basis of racial blood pressure differences. Hypertension 1, 468–475 (1979)
Greeley, H. T. Pharmacogenomics: Promise, prospects, and potential problems. Medical Ethics 9, 1–2, 8 (2002) (link to article)
Liggett, S. B., et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nature Medicine 14, 510–517 (2008) doi:10.1038/nm1750 (link to article)
Meyer, U. A. Pharmacogenetics—Five decades of therapeutic lessons from genetic diversity. Nature Reviews Genetics 5, 669–676 (2004) doi:10.1038/nrg1428 (link to article)
Sankar, P., et al. Genetic research and health disparities. Journal of the American Medical Association 291, 2985–2989 (2004)
———. Race and ethnicity in genetic research. American Journal of Medical Genetics 143A, 961–970 (2007)
Saunders, E., & Gavin, J. R. Blockade of the renin-angiotensin system in African Americans with hypertension and cardiovascular disease. Journal of Clinical Hypertension 5, 12–17 (2003) doi:10.1111/j.1524-6175.2003.02181.
Tang, H., et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics 76, 268–275 (2005)
Wilson, J. F., et al. Population genetic structure of variable drug response. Nature Genetics 29, 265–269 (2001) doi:10.1038/ng761 (link to article)
Yancy, C. W., et al. Race and the response to adrenergic blockade with carvedilol in patients with chronic heart failure. New England Journal of Medicine 344, 1358–1365 (2001)
Zanger, U. M., et al. Cytochrome P450 2D6: Overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedeberg's Archives of Pharmacology 369, 23–37 (2003)



 Table 1: Proportion of membership of each sampled population in structure-defined subclusters
Table 1: Proportion of membership of each sampled population in structure-defined subclusters


























