« Prev Next »
How Proteins Fold
All of the information required to build a living organism is stored in its chromosomes, and the end result of the expression of most genes in those chromosomes is a protein. Proteins play crucial roles in essentially all biological processes. When you observe a cell through the lens of a microscope, what you are really seeing are proteins in action. There are proteins that give the cell its form and structure, whereas other proteins perform all the vital functions required for the cell to operate. Proteins can function as catalysts (changing the rate of chemical reactions), in the transport and storage of other molecules (such as oxygen or iron), and in immune protection, among many other functions. They are able to generate movement, transmit nerve impulses through membrane receptors, act as hormones, and provide control of growth and differentiation.
In their most basic state, proteins are composed of a linear chain of amino acids, and these linear molecules normally fold up into specific three-dimensional shapes. A protein's function directly depends on its folded structure. Additionally, each protein contains a wide range of functional groups (groups of atoms attached to the amino acids that participate in specific chemical reactions) that may affect its final shape.
Functional groups are added to the protein after it is synthesized in the ribosomes in a process called post-translational modification, which takes place in the endoplasmic reticulum. What are these post-translational modifications for? These chemical modifications contribute to the proper folding of proteins, promote their activation from inactive forms, affect their functions, determine their cellular localizations, or mark them for secretion. These modifications affect the overall condition of proteins within the cell and/or the organism. Post-translational modifications include: the addition of phosphate groups (phosphorylation), sugars (glycosylation), fats (lipidation), acetyl groups (acetylation), and the breaking of proteins into fragments (proteolysis). There are more than 100 different types of these modifications, reflecting the importance of post-translational modification in protein functions.
Some proteins do not fold into their correct three dimensional structures by themselves. They need help. Specialized proteins, collectively known as chaperones, come to their rescue. The mission of the chaperones is to help other proteins fold into their correct configuration and thus acquire functionality (Herbert & Molinari 2007).
The Discovery of Calreticulin: Many Names, One Protein
Calreticulin was discovered in skeletal muscle cells in 1974. Thomas Ostwald and David MacLennan were characterizing proteins that bind to calcium in the sarcoplasmic reticulum — a specialized type of endoplasmic reticulum found in muscle cells. Since calcium is essential for muscle contraction and relaxation, they hoped to learn about the regulation of calcium by studying these proteins in muscle cells. Ostwald and MacLennan isolated seven proteins from an extract of rabbit skeletal muscle sarcoplasmic reticulum, and when they measured the calcium binding activity, only one protein showed a high affinity for the element. They gave this newly identified protein the name "high affinity calcium binding protein" (HABP), for obvious reasons.
Fifteen years later, advances in molecular biology allowed scientists to clone and characterize the amino acid sequence of HABP (Fliegel et al. 1989). To their surprise, when they compared its amino acid sequence to those of other calcium binding proteins reported in the literature, they learned that the sequence was identical to those of calregulin, CRP55, CaBP3, ERp60, and calsequestrin-like protein (Michalak et al. 1992). Out of all these proteins, CRP55 was the first sequenced and proposed to be involved in calcium storage, a prediction that turned out to be correct. The authors named this protein calreticulin (Smith & Koch 1989). Previously, calregulin had been localized in the endoplasmic reticulum (Waisman et al. 1985), and calreticulin in the luminal material of that organelle (Macer and Koch 1988). All of these proteins had been discovered by different research groups studying a variety of non-muscle cells, such as liver and brain cells. To avoid confusion, all of the laboratories involved reached a consensus to name the protein "calreticulin," reflecting its calcium binding capacity and its localization in the sarcoplasmic/endoplasmic reticulum. At the time, they had no idea that this multi-named protein would also turn out to be a multifaceted molecule. Next, scientists compared the human calreticulin (CRT) amino acid sequence with other known sequences from different organisms using phylogenetic analyses. Their results showed that CRT is a conserved protein, suggesting that it plays an essential role in cell physiology. So far, CRT has been found in all eukaryotic cells, except erythrocytes — these cells lack an ER — and yeast (Michalak 1999).
Once scientists have some knowledge about the structure of a protein, they can then make predictions about the protein's functions. The structure of CRT suggested that it has two main functions: i) CRT acts as a chaperone helping other proteins fold correctly and become functional proteins (this is considered a characteristic quality control process of the endoplasmic reticulum), and ii) CRT regulates intracellular calcium levels, acting as a sensor and buffer of this element in the ER lumen.
Once the scientists made these predictions, they had to design experiments to examine their hypotheses. How did they show that CRT was a chaperone and that the protein participated in calcium metabolism? To test these hypotheses required many years of hard work and painstaking experiments.
The Life of CRT as a Chaperone
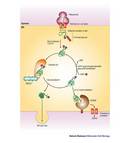
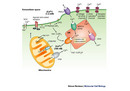
When a polypeptide is incorrectly folded, it is bound by one or more chaperones (including CRT) and retained in the endoplasmic reticulum. The chaperones help the polypeptide fold into the correct configuration. If the polypeptide fails to fold properly, it will not be transported to its final destination. Instead, it will be transferred from the endoplasmic reticulum back to the cytoplasm where it will be degraded by the proteasome, a multi-protein complex where proteins are broken down into fragments. This mechanism prevents the expression, aggregation, and secretion of misfolded proteins, which can be detrimental to the cell. If the newly synthesized protein is properly folded, it will be transported to the Golgi complex and secreted. As a final option, if the polypeptide does not achieve its native conformation and still has unfolded domains, it can be re-bound by CRT, giving it another chance to fold into the proper conformation (Figure 2).
CRT Participates in Calcium Metabolism
Their experiments showed that the CRT gene was active during the early stages of cardiac development, since they detected a fluorescent signal in the embryo's heart. However, they observed that the CRT gene switched off in mature cardiac tissue. In addition, they noted the key finding that cells deficient in CRT were unable to release calcium from the endoplasmic reticulum into the cytosol upon stimulation. The scientists concluded that the impairment in cardiac development was due to the CRT's calcium regulating function (Figure 3).
Multi-Functionality
Besides the essential functions CRT performs in the ER lumen, this multifaceted protein has also been implicated in many completely unexpected roles that occur at the cell surface, in the cytosol, within the nucleus, and in the extracellular matrix. Surprisingly, CRT has been detected in all of these cellular regions. Indeed, since the discovery of CRT's chaperone and calcium-regulating functions, scientists have learned that CRT has many other duties in the cell. CRT has been implicated in diverse cellular processes including signaling, regulation of gene expression, cell adhesion, apoptosis (programmed cell death), wound healing, removal of cancer cells, and autoimmunity. Now that is multi-functionality!
Calreticulin in Parasite Infections: A Personal Encounter
Since CRT has so many functions, we immediately wondered what role it could play in T. solium. As expected from its known functions, we observed that CRT from T. solium binds calcium. We also observed that CRT expression is developmentally regulated in the parasite. We used immunological techniques to identify the location of CRT. By using antibodies that specifically recognized CRT, we were able to demonstrate that CRT is mainly present during germ cell development and embryogenesis. However, its expression is strictly regulated, both spatially and temporally. CRT is expressed in the germinal cells, but once fertilization takes place, its expression starts to diminish, until it is practically absent in the infective egg. These findings led us to hypothesize that CRT had an important role in parasite development, and that CRT might be used to generate a vaccine to interfere with the tapeworms' growth.
| Table 1. Calreticulin functions in parasites that cause disease in humans | |||
| Parasite | Disease |
CRT Localization |
CRT Function
|
| Entamoeba histolytica | Amibiasis | ER, cell surface, uropod | Phagocytosis |
| Trypanosoma cruzi | Chagas disease | ER, cell surface | Lectin-like chaperone, interaction with C1q, recognized by IgG from patients |
| Leishmania donovani | Visceral leishmaniasis | Somatic and secreted forms | Involved in secretion of acid phosphatases |
| Necator americanus | Hookworm infection | Somatic and secreted forms | Allergen, induces basophil histamine release, interaction with C1q |
| Schistosoma mansoni | Schistosomiasis | Somatic and secreted forms, penetration glands | Regulation of Ca2+ dependent proteases involved in skin penetration and migration, T and B cell immunogen |
| Taenia solium | Taeniosis and neurocysticercosis | Somatic and secreted forms, gametes and developing embryos | Possible role in egg development |
After several vaccination trials in a hamster experimental model, we observed that vaccinated animals contained fewer parasites than unvaccinated control animals. Most importantly, the parasites were unable to mature in the vaccinated animals (Mendlovic 2004, 2006, Leon-Cabrera 2009). CRT is not unique to T. solium. It has also been described in several other parasites (Table 1). We still have many questions concerning CRT in T. solium. For instance, is it secreted by the parasite? If so, could it interact with immune cells from the host? What are the mechanisms responsible for the impaired development of the parasite following vaccination? We are currently trying to answer these questions.
Summary
Scientists have just begun to understand the multiple facets of CRT functions, and there are more questions than answers. Recently, scientists showed that when CRT was applied directly to the surface of cutaneous wounds, it induced cell proliferation and resulted in faster healing (Gold et al. 2010). Scientists have also identified alternative forms of the protein (isoforms) in both mammals and plants, in addition to different post-translationally modified forms (Michalak 1999). These characteristics, together with the fact that CRT modulates calcium signaling and is involved in correctly folding many proteins, may explain the plethora of functions that are regulated by this multifaceted protein. CRT is a protein with such extraordinary characteristics that will certainly continue to puzzle researchers for years to come.
References and Recommended Reading
Fliegel, L., Burns, K., et al. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. Journal of Biological Chemistry 264, 21522–21528 (1989).
Gold, L. I., Eggleton, P., et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB Journal 24, 665–683 (2010).
Herbert D, N. & Molinari M. In and out of the ER: protein folding, quality control, degradation and related diseases. Physiological Reviews 87, 1377–1408 (2007).
León-Cabrera, S., Cruz-Rivera, M., et al. Standardization of an experimental model of human taeniosis for oral vaccination. Methods. 49, 346–350 (2009).
Macer, D. R. & Koch, G. L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. Journal of Cell Science 91, 61–70 (1988).
Mendlovic, F., Ostoa-Saloma, P., et al. Cloning, characterization, and functional expression of Taenia solium calreticulin. Journal of Parasitology 90, 891–893 (2004).
Mendlovic, F., Carillo-Farga, J., et al. Differential expression of calreticulin in developmental stages of Taenia solium. Journal of Parasitology 92, 789–795 (2006).
Mesaeli, N., Nakamura, K., et al. Calreticulin is essential for cardiac development. Journal of Cell Biology 144, 857–868 (1999).
Michalak, M., Corbett, E. F., et al. Calreticulin: one protein, one gene, many functions. Biochemical Journal 344, 281–292 (1999).
Michalak, M., Milner, R. E., et al. Calreticulin. Biochemical Journal 285, 681–692 (1992).
Ostwald, T. J. & MacLennan, D. H. Isolation of a high affinity calcium-binding protein for sarcoplasmic reticulum. Journal of Biological Chemistry 249, 974–979 (1974).
Peterson, J. R., Ora, A., et al. Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Molecular Biology of the Cell 6, 1173–1184 (1995).
Smith, M. J. & Koch, G.L. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding ER/SR protein. EMBO Journal 8, 3581–3586 (1989).
Waisman, D.M., Salimath, B.P., et al. Isolation and characterization of CAB-63, a novel calcium-binding protein. The Journal of Biological Chemistry 260, 1652–1660 (1985).




 Figure 1: Structure and functions of the calreticulin domains
Figure 1: Structure and functions of the calreticulin domains