« Prev Next »
Perhaps the most amazing thing about mitosis is its precision, a feature that has intrigued biologists since Walther Flemming first described chromosomes in the late 1800s (Paweletz, 2001). Although Flemming was able to correctly deduce the sequence of events in mitosis, this sequence could not be experimentally verified for several decades, until advances in light microscopy made it possible to observe chromosome movements in living cells. Researchers now know that mitosis is a highly regulated process involving hundreds of different cellular proteins. The dynamic nature of mitosis is best appreciated when this process is viewed in living cells.
Mitosis Occupies a Portion of the Cell Cycle
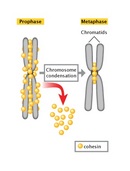
The difference in DNA compaction between interphase and mitosis is dramatic. A precise estimate of the difference is not possible, but during interphase, chromatin may be hundreds or even thousands of times less condensed than it is during mitosis. For this reason, the enzyme complexes that copy DNA have the greatest access to chromosomal DNA during interphase, at which time the vast majority of gene transcription occurs. In addition, chromosomal DNA is duplicated during a subportion of interphase known as the S, or synthesis, phase. As the two daughter DNA strands are produced from the chromosomal DNA during S phase, these daughter strands recruit additional histones and other proteins to form the structures known as sister chromatids (Figure 2). The sister chromatids, in turn, become "glued" together by a protein complex named cohesin. Cohesin is a member of the SMC, or structural maintenance of chromosomes, family of proteins. SMC proteins are DNA-binding proteins that affect chromosome architectures; indeed, cells that lack SMC proteins show a variety of defects in chromosome stability or chromosome behavior. Current data suggest that cohesin complexes may literally form circles that encompass the two sister chromatids (Hirano, 2002; Hagstrom & Meyer, 2003). At the end of S phase, cells are able to sense whether their DNA has been successfully copied, using a complicated set of checkpoint controls that are still not fully understood. For the most part, only cells that have successfully copied their DNA will proceed into mitosis.
Chromatin Is Extensively Condensed as Cells Enter Mitosis
The most obvious difference between interphase and mitosis involves the appearance of a cell's chromosomes. During interphase, individual chromosomes are not visible, and the chromatin appears diffuse and unorganized. Recent research suggests, however, that this is an oversimplification and that chromosomes may actually occupy specific territories within the nucleus (Cremer & Cremer, 2001). In any case, as mitosis begins, a remarkable condensation process takes place, mediated in part by another member of the SMC family, condensin (Hirano, 2002; Hagstrom & Meyer, 2003). Like cohesin, condensin is an elongated complex of several proteins that binds and encircles DNA. In contrast to cohesin, which binds two sister chromatids together, condensin is thought to bind a single chromatid at multiple spots, twisting the chromatin into a variety of coils and loops (Figure 3).
The Mitotic Spindle Aids in Chromosome Separation
During mitosis, chromosomes become attached to the structure known as the mitotic spindle. In the late 1800s, Theodor Boveri created the earliest detailed drawings of the spindle based on his observations of cell division in early Ascaris embryos (Figure 4; Satzinger, 2008). Boveri's drawings, which are amazingly accurate, show chromosomes attached to a bipolar network of fibers. Boveri observed that the spindle fibers radiate from structures at each pole that we now recognize as centrosomes, and he also noted that each centrosome contains two small, rodlike bodies, which are now known as centrioles. Boveri observed that the centrioles duplicate before the chromosomes become visible and that the two pairs of centrioles move to separate poles before the spindle assembles. We now know that centrioles duplicate during S phase, although many details of this duplication process are still under investigation.
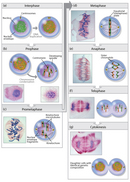
Mitosis Is Divided into Well-Defined Phases
From his many detailed drawings of mitosen, Walther Flemming correctly deduced, but could not prove, the sequence of chromosome movements during mitosis (Figure 7). Flemming divided mitosis into two broad parts: a progressive phase, during which the chromosomes condensed and aligned at the center of the spindle, and a regressive phase, during which the sister chromatids separated. Our modern understanding of mitosis has benefited from advances in light microscopy that have allowed investigators to follow the process of mitosis in living cells. Such live cell imaging not only confirms Flemming's observations, but it also reveals an extremely dynamic process that can only be partially appreciated in still images.
Prophase
Mitosis begins with prophase, during which chromosomes recruit condensin and begin to undergo a condensation process that will continue until metaphase. In most species, cohesin is largely removed from the arms of the sister chromatids during prophase, allowing the individual sister chromatids to be resolved. Cohesin is retained, however, at the most constricted part of the chromosome, the centromere (Figure 9). During prophase, the spindle also begins to form as the two pairs of centrioles move to opposite poles and microtubules begin to polymerize from the duplicated centrosomes.
Prometaphase
Prometaphase begins with the abrupt fragmentation of the nuclear envelope into many small vesicles that will eventually be divided between the future daughter cells. The breakdown of the nuclear membrane is an essential step for spindle assembly. Because the centrosomes are located outside the nucleus in animal cells, the microtubules of the developing spindle do not have access to the chromosomes until the nuclear membrane breaks apart.
Prometaphase is an extremely dynamic part of the cell cycle. Microtubules rapidly assemble and disassemble as they grow out of the centrosomes, seeking out attachment sites at chromosome kinetochores, which are complex platelike structures that assemble during prometaphase on one face of each sister chromatid at its centromere. As prometaphase ensues, chromosomes are pulled and tugged in opposite directions by microtubules growing out from both poles of the spindle, until the pole-directed forces are finally balanced. Sister chromatids do not break apart during this tug-of-war because they are firmly attached to each other by the cohesin remaining at their centromeres. At the end of prometaphase, chromosomes have a bi-orientation, meaning that the kinetochores on sister chromatids are connected by microtubules to opposite poles of the spindle.
Metaphase
Next, chromosomes assume their most compacted state during metaphase, when the centromeres of all the cell's chromosomes line up at the equator of the spindle. Metaphase is particularly useful in cytogenetics, because chromosomes can be most easily visualized at this stage. Furthermore, cells can be experimentally arrested at metaphase with mitotic poisons such as colchicine. Video microscopy shows that chromosomes temporarily stop moving during metaphase. A complex checkpoint mechanism determines whether the spindle is properly assembled, and for the most part, only cells with correctly assembled spindles enter anaphase.
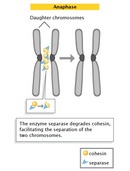
Anaphase
The progression of cells from metaphase into anaphase is marked by the abrupt separation of sister chromatids. A major reason for chromatid separation is the precipitous degradation of the cohesin molecules joining the sister chromatids by the protease separase (Figure 10).
Two separate classes of movements occur during anaphase. During the first part of anaphase, the kinetochore microtubules shorten, and the chromosomes move toward the spindle poles. During the second part of anaphase, the spindle poles separate as the non-kinetochore microtubules move past each other. These latter movements are currently thought to be catalyzed by motor proteins that connect microtubules with opposite polarity and then "walk" toward the end of the microtubules.
Telophase and Cytokinesis
Mitosis ends with telophase, or the stage at which the chromosomes reach the poles. The nuclear membrane then reforms, and the chromosomes begin to decondense into their interphase conformations. Telophase is followed by cytokinesis, or the division of the cytoplasm into two daughter cells. The daughter cells that result from this process have identical genetic compositions.
References and Recommended Reading
Cheeseman, I. M., & Desai, A. Molecular architecture of the kinetochore-microtubule interface. Nature Reviews Molecular Cell Biology 9, 33–46 (2008) doi:10.1038/nrm2310 (link to article)
Cremer, T., & Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nature Reviews Genetics 2, 292–301 (2001) doi:10.1038/35066075 (link to article)
Hagstrom, K. A., & Meyer, B. J. Condensin and cohesin: More than chromosome compactor and glue. Nature Reviews Genetics 4, 520–534 (2003) doi:10.1038/nrg1110 (link to article)
Hirano, T. At the heart of the chromosome: SMC proteins in action. Nature Reviews Molecular Cell Biology 7, 311–322 (2002) doi:10.1038/nrm1909 (link to article)
Mitchison, T. J., & Salmon, E. D. Mitosis: A history of division. Nature Cell Biology 3, E17–E21 (2001) doi:10.1038/35050656 (link to article)
Paweletz, N. Walther Flemming: Pioneer of mitosis research. Nature Reviews Molecular Cell Biology 2, 72–75 (2001) doi:10.1038/35048077 (link to article)
Satzinger, H. Theodor and Marcella Boveri: Chromosomes and cytoplasm in heredity and development. Nature Reviews Genetics 9, 231–238 (2008) doi:10.1038.nrg2311 (link to article)




 Figure 2
Figure 2