« Prev Next »
Previous Section: What Controls Ocean Productivity on Long Time Scales?
How Does Ocean Productivity Affect Atmospheric Carbon Dioxide?
On the time scale of thousands of years, the chemistry of the ocean essentially sets the concentration of CO2 in the atmosphere (Broecker 1982). Ocean productivity affects atmospheric CO2 by the export of both organic carbon and calcium carbonate (CaCO3) from the surface ocean to depth; the former lowers atmospheric CO2, while the latter raises it more modestly (Archer 2003, Sarmiento & Gruber 2006). These opposing effects on CO2 are evident at a simplistic level from the reactions associated with (1) the formation of organic carbon (as CH2O, sugar) that consumes dissolved CO2 and (2) the precipitation of CaCO3 that releases it:
Organic carbon: CO2 + H2O → CH2O + O2 (eq. 1)
Calcium carbonate: Ca2+ + 2HCO3- → CaCO3 + CO2 (eq. 2)
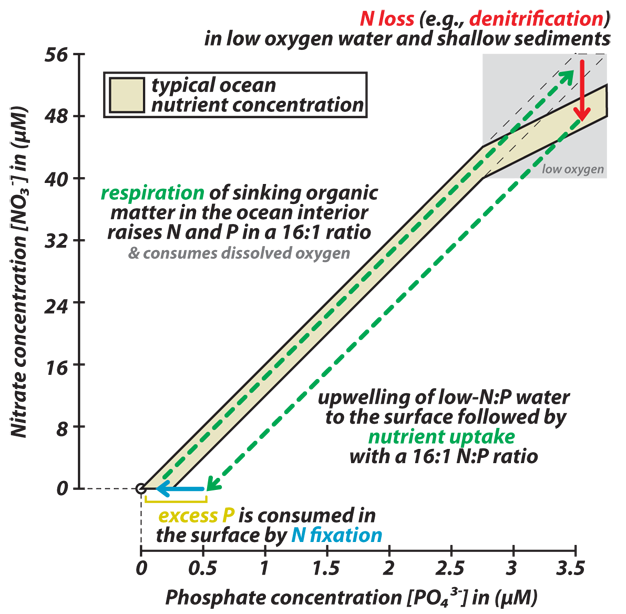
The downward transport of organic carbon extracts dissolved inorganic carbon from the surface ocean and atmosphere, sequestering it in the deep sea. This "biological pump" for carbon is coupled to the removal of nutrients (e.g., N and P) from surface waters and their accumulation in the deep ocean, as described above. However, in the case of dissolved inorganic carbon — the sum of three inorganic carbon species: dissolved CO2, bicarbonate (HCO3-), and carbonate (CO32-) — only ~10% of it is consumed from surface waters, while N and P consumption is often effectively complete. The term "biological pump" is sometimes replaced with the term "soft tissue pump" to explicitly specify the impact of organic carbon rain out of the surface ocean and its subsequent degradation in deep waters, to be distinguished from the "carbonate pump," in which CaCO3 is precipitated in surface waters and exported to depth.
The soft-tissue pump of carbon from the surface to the interior lowers atmospheric CO2 directly by simply shuttling carbon out of surface waters, which causes CO2 from the atmosphere to invade the surface ocean. In addition, the soft tissue pump, by pumping the weak acid CO2 into deep water, works to lower the pH of the deep ocean. This forces the whole ocean to become more alkaline to bury the requisite amount of CaCO3 to balance river inputs from rock weathering on land (see below). Since alkaline solutions can hold more dissolved inorganic carbon by deprotonating carbonic acid (H2CO3, or CO2 + H2O) to bicarbonate and carbonate, this causes a further decline in atmospheric CO2.
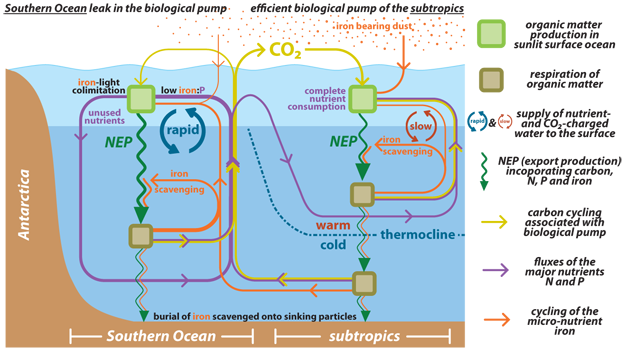
The overall strength the soft tissue pump is determined by (1) the major nutrient content of the ocean, (2) the degree to which the major nutrients are consumed in surface waters, and (3) the carbon-to-major nutrient ratio of sinking organic matter. Calculations suggest that, in the context of the modern carbon cycle, if the soft tissue pump were to stop, atmospheric CO2 concentration would more than double over the course of roughly a thousand years (the time scale over which deep waters pass through the surface ocean). Conversely, a fully efficient soft tissue pump (one in which all N and P supplied to surface waters is consumed and converted into exported organic matter) would lower CO2 by more than half of its current concentration. Given this potential for further CO2 drawdown, an increase in the efficiency of the soft-tissue pump (from about ~50% efficiency today to ~75% efficiency) may explain the reduction in atmospheric CO2 concentrations that is observed to have occurred during past ice ages. The above estimates are simplifications in that a change in ocean productivity would likely also affect CaCO3 cycling. For example, without ocean productivity, CaCO3 would not be produced by organisms in the surface ocean, and this CaCO3 production raises CO2 (see below).
Marine plankton such as coccolithophores (phytoplankton) and foraminifera (zooplankton) produce CaCO3 hard parts. Dissolved CaCO3 is a base; therefore, its precipitation from solution removes alkalinity and lowers the pH of seawater. The lower pH, in turn, converts more of seawater's dissolved inorganic carbon into the (acidic) CO2 form. Thus, when CaCO3 is precipitated in surface waters and exported from them, it raises the CO2 concentration of the surface ocean (eq. 2) and drives a CO2 flux into the atmosphere.
Carbonates are much denser than seawater and sink rapidly into the deep ocean, where, under the pressure of the water column and the lower pH caused by the breakdown of organic C from the soft-tissue pump, they become chemically unstable and some of the CaCO3 dissolves. The CaCO3 that is preserved and buried on the seafloor represents the single biggest continuous loss of alkalinity from ocean water, balancing the input of alkalinity from the weathering of carbonate and silicate rocks on land. If it were not for biological precipitation of CaCO3, the ocean would need to have a higher pH in order to spontaneously precipitate CaCO3 and balance the input of alkalinity from weathering; the higher pH would lower atmospheric CO2. Thus, both with regard to the internal cycling of CaCO3 in the ocean and the whole ocean's input/output budget of alkalinity, biological CaCO3 production works to raise atmospheric CO2. However, the soft-tissue pump can manipulate the CaCO3 cycle: strengthening the soft-tissue pump lowers the pH of the deep ocean and reduces deep sea CaCO3 burial, forcing the whole ocean to gain alkalinity, which works to lower atmospheric CO2. Finally, there is no fundamental need for ocean productivity to produce CaCO3, so the relative proportions of CaCO3 and organic carbon rain generated by ocean productivity may vary greatly, with potential consequences for CO2. A full exposition of this complex system is provided by Archer (2003) and Sarmiento & Gruber (2006).
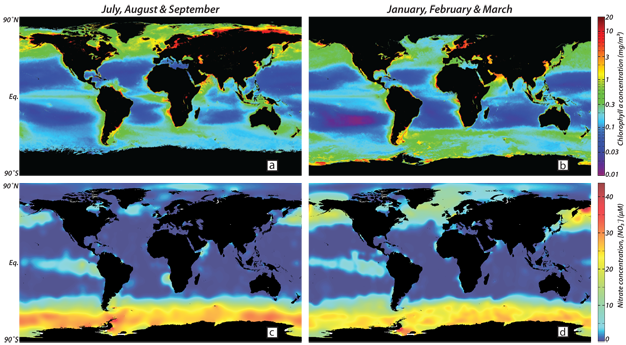
To address a common misconception, the capacity of ocean productivity to lower atmospheric CO2 is not typically made stronger by simply increasing ocean upwelling rates. Increased upwelling increases the nutrient supply for productivity, but also exposes to the atmosphere the CO2 previously sequestered by the soft tissue pump. In the low latitude ocean, these effects roughly offset one another. Productivity is highest in the polar regions (Figure 4), and yet the incompleteness of nutrient consumption in these regions causes them to release biologically sequestered CO2 back to the atmosphere (Figure 5). For a given concentration of the ocean's major nutrients, it is the completeness of nutrient consumption rather than the rate of organic matter export that matters for CO2 sequestration. This is true up to the time scale of 100 thousand years or more, at which point the rate of organic matter export does become important because the slow burial of organic carbon is a significant mechanism of carbon removal.
In the Following Section: Are Humans Changing Ocean Productivity?

































