« Prev Next »

Introduction
Organisms live within an ecological community, which is defined as an assemblage of populations of at least two different species that interact directly and indirectly within a defined geographic area (Agrawal et al. 2007; Ricklefs 2008; Brooker et al. 2009). Species interactions form the basis for many ecosystem properties and processes such as nutrient cycling and food webs. The nature of these interactions can vary depending on the evolutionary context and environmental conditions in which they occur. As a result, ecological interactions between individual organisms and entire species are often difficult to define and measure and are frequently dependent on the scale and context of the interactions (Harrison & Cornell 2008; Ricklefs 2008; Brooker et al. 2009). Nonetheless, there are several classes of interactions among organisms that are found throughout many habitats and ecosystems. Using these classes of interactions as a framework when studying an ecological community allows scientists to describe naturally occurring processes and aids in predicting how human alterations to the natural world may affect ecosystem properties and processes.At the coarsest level, ecological interactions can be defined as either intra-specific or inter-specific. Intra-specific interactions are those that occur between individuals of the same species, while interactions that occur between two or more species are called inter-specific interactions. However, since most species occur within ecological communities, these interactions can be affected by, and indirectly influence, other species and their interactions. The ones that will be discussed in this article are competition, predation, herbivory and symbiosis. These are not the only types of species interactions, just the most studied — and they are all parts of a larger network of interactions that make up the complex relationships occurring in nature.
Competition
Competition is most typically considered the interaction of individuals that vie for a common resource that is in limited supply, but more generally can be defined as the direct or indirect interaction of organisms that leads to a change in fitness when the organisms share the same resource. The outcome usually has negative effects on the weaker competitors. There are three major forms of competition. Two of them, interference competition and exploitation competition, are categorized as real competition. A third form, apparent competition, is not. Interference competition occurs directly between individuals, while exploitation competition and apparent competition occur indirectly between individuals (Holomuzki et. al 2010) (Figure 1).
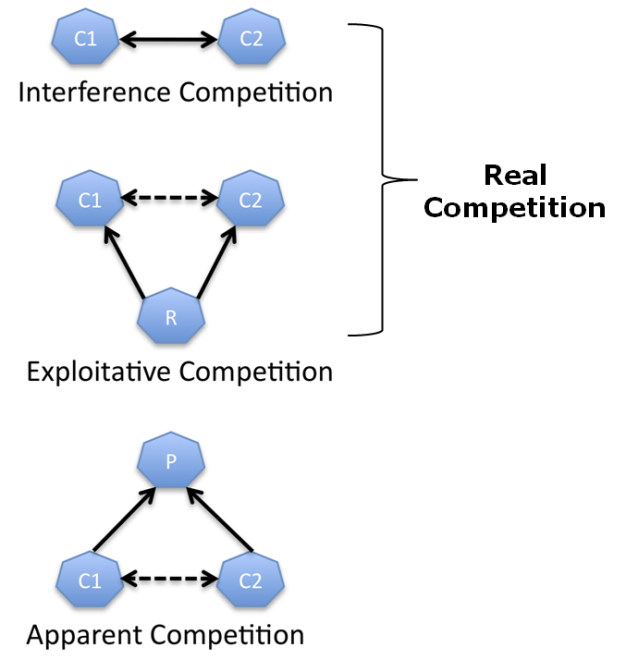
When an individual directly alters the resource-attaining behavior of other individuals, the interaction is considered interference competition. For example, when a male gorilla prohibits other males from accessing a mate by using physical aggression or displays of aggression, the dominant male is directly altering the mating behavior of other males. This is also an example of an intra-specific interaction. Exploitation competition occurs when individuals interact indirectly as they compete for common resources, like territory, prey or food. Simply put, the use of the resource by one individual will decrease the amount available for other individuals. Whether by interference or exploitation, over time a superior competitor can eliminate an inferior one from the area, resulting in competitive exclusion (Hardin 1960). The outcomes of competition between two species can be predicted using equations, and one of the most well known is the Lotka-Volterra model (Volterra 1926, Lotka 1932). This model relates the population density and carrying capacity of two species to each other and includes their overall effect on each other. The four outcomes of this model are: 1) species A competitively excludes species B; 2) species B competitively excludes species A; 3) either species wins based on population densities; or 4) coexistence occurs. Species can survive together if intra-specific is stronger than inter-specific competition. This means that each species will inhibit their own population growth before they inhibit that of the competitor, leading to coexistence.
Another mechanism for avoiding competitive exclusion is to adopt alternative life history and dispersal strategies, which are usually reinforced through natural selection. This mechanism reduces competitive interactions and increases opportunities for new colonization and nutrient acquisition. The success of this is often dependent upon events (such as tide, flood, or fire disturbances) that create opportunities for dispersal and nutrient acquisition. Consider that Plant Species A is more efficient than Plant Species B at nutrient uptake, but Plant B is a better disperser. In this example, the resource under competition is nutrients, but nutrient acquisition is related to availability. If a disturbance opens up new space for colonization, Plant B is expected to arrive first and maintain its presence in the community until Plant A arrives and begins competing with Plant B. Eventually Plant A will outcompete Plant B, perhaps by growing faster because Plant A is more efficient at nutrient acquisition. With an increasing Plant A population, the Plant B population will decline, and given enough time, can be excluded from that area. The exclusion of Plant B can be avoided if a local disturbance (for example, prairie fires) consistently opens new opportunities (space) for colonization. This often happens in nature, and thus disturbance can balance competitive interactions and prevent competitive exclusion by creating patches that will be readily colonized by species with better dispersal strategies (Roxburgh et al. 2004) (Figure 2). The success of the dispersal versus nutrient acquisition trade-off depends, however, on the frequency and spatial proximity (or how close they are) of disturbance events relative to the dispersal rates of individuals of the competing species. Coexistence can be achieved when disturbances occur at a frequency or distance that allows the weaker, but often better dispersing, competitor to be maintained in a habitat. If the disturbance is too frequent the inferior competitor (better disperser) wins, but if the disturbance is rare then the superior competitor slowly outcompetes the inferior competitor, resulting in competitive exclusion. This is known as the intermediate disturbance hypothesis (Horn 1975, Connell 1978).

Apparent competition occurs when two individuals that do not directly compete for resources affect each other indirectly by being prey for the same predator (Hatcher et al. 2006). Consider a hawk (predator, see below) that preys both on squirrels and mice. In this relationship, if the squirrel population increases, then the mouse population may be positively affected since more squirrels will be available as prey for the hawks. However, an increased squirrel population may eventually lead to a higher population of hawks requiring more prey, thus, negatively affecting the mice through increased predation pressure as the squirrel population declines. The opposite effect could also occur through a decrease in food resources for the predator. If the squirrel population decreases, it can indirectly lead to a reduction in the mouse population since they will be the more abundant food source for the hawks. Apparent competition can be difficult to identify in nature, often because of the complexity of indirect interactions that involve multiple species and changing environmental conditions.
Predation and Herbivory
Predation requires one individual, the predator, to kill and eat another individual, the prey (Figure 3). In most examples of this relationship, the predator and prey are both animals; however, protozoans are known to prey on bacteria and other protozoans and some plants are known to trap and digest insects (for example, pitcher plant) (Figure 4). Typically, this interaction occurs between species (inter-specific); but when it occurs within a species (intra-specific) it is cannibalism. Cannibalism is actually quite common in both aquatic and terrestrial food webs (Huss et al. 2010; Greenwood et al. 2010). It often occurs when food resources are scarce, forcing organisms of the same species to feed on each other. Surprisingly, this can actually benefit the species (though not the prey) as a whole by sustaining the population through times of limited resources while simultaneously allowing the scarce resources to rebound through reduced feeding pressure (Huss et al. 2010). The predator-prey relationship can be complex through sophisticated adaptations by both predators and prey, in what has been called an "evolutionary arms race." Typical predatory adaptations are sharp teeth and claws, stingers or poison, quick and agile bodies, camouflage coloration and excellent olfactory, visual or aural acuity. Prey species have evolved a variety of defenses including behavioral, morphological, physiological, mechanical, life-history synchrony and chemical defenses to avoid being preyed upon (Aaron, Farnsworth et al. 1996, 2008).


Another interaction that is much like predation is herbivory, which is when an individual feeds on all or part of a photosynthetic organism (plant or algae), possibly killing it (Gurevitch et al. 2006). An important difference between herbivory and predation is that herbivory does not always lead to the death of the individual. Herbivory is often the foundation of food webs since it involves the consumption of primary producers (organisms that convert light energy to chemical energy through photosynthesis). Herbivores are classified based on the part of the plant consumed. Granivores eat seeds; grazers eat grasses and low shrubs; browsers eat leaves from trees or shrubs; and frugivores eat fruits. Plants, like prey, also have evolved adaptations to herbivory. Tolerance is the ability to minimize negative effects resulting from herbivory, while resistance means that plants use defenses to avoid being consumed. Physical (for example, thorns, tough material, sticky substances) and chemical adaptations (for example, irritating toxins on piercing structures, and bad-tasting chemicals in leaves) are two common types of plant defenses (Gurevitch et al. 2006) (Figure 5).

Symbiosis: Mutualism, Commensalism and Parasitism
Symbiosis is an interaction characterized by two or more species living purposefully in direct contact with each other. The term "symbiosis" includes a broad range of species interactions but typically refers to three major types: mutualism, commensalism and parasitism. Mutualism is a symbiotic interaction where both or all individuals benefit from the relationship. Mutualism can be considered obligate or facultative. (Be aware that sometimes the term "symbiosis" is used specifically to mean mutualism.) Species involved in obligate mutualism cannot survive without the relationship, while facultative mutualistic species can survive individually when separated but often not as well (Aaron et al. 1996). For example, leafcutter ants and certain fungi have an obligate mutualistic relationship. The ant larvae eat only one kind of fungi, and the fungi cannot survive without the constant care of the ants. As a result, the colonies activities revolve around cultivating the fungi. They provide it with digested leaf material, can sense if a leaf species is harmful to the fungi, and keep it free from pests (Figure 6). A good example of a facultative mutualistic relationship is found between mycorrhizal fungi and plant roots. It has been suggested that 80% of vascular plants form relationships with mycorrhizal fungi (Deacon 2006). Yet the relationship can turn parasitic when the environment of the fungi is nutrient rich, because the plant no longer provides a benefit (Johnson et al. 1997). Thus, the nature of the interactions between two species is often relative to the abiotic conditions and not always easily identified in nature.

Commensalism is an interaction in which one individual benefits while the other is neither helped nor harmed. For example, orchids (examples of epiphytes) found in tropical rainforests grow on the branches of trees in order to access light, but the presence of the orchids does not affect the trees (Figure 7). Commensalism can be difficult to identify because the individual that benefits may have indirect effects on the other individual that are not readily noticeable or detectable. If the orchid from the previous example grew too large and broke off the branch or shaded the tree, then the relationship would become parasitic.

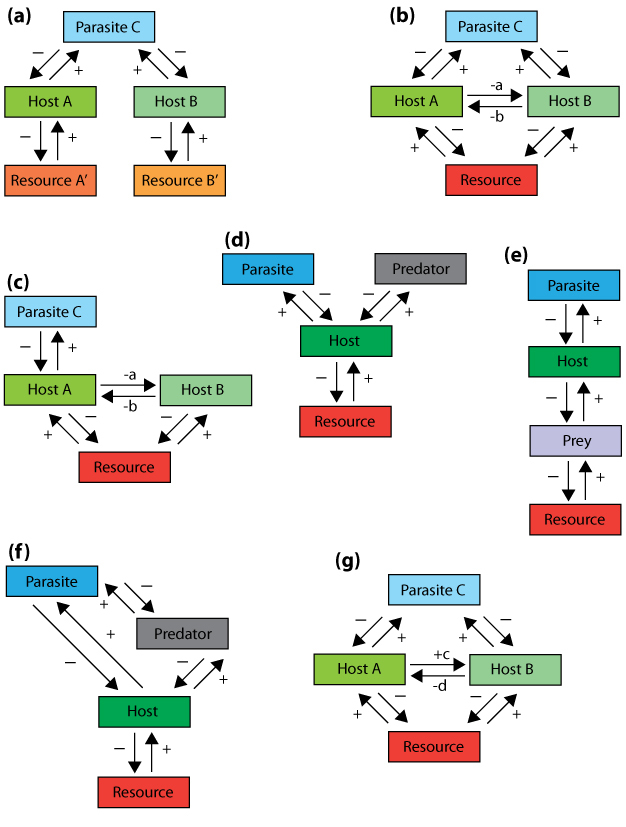
Parasitism occurs when one individual, the parasite, benefits from another individual, the host, while harming the host in the process. Parasites feed on host tissue or fluids and can be found within (endoparasites) or outside (ectoparasites) of the host body (Holomuzki et al. 2010). For example, different species of ticks are common ectoparasites on animals and humans. Parasitism is a good example of how species interactions are integrated. Parasites typically do not kill their hosts, but can significantly weaken them; indirectly causing the host to die via illness, effects on metabolism, lower overall health and increased predation potential (Holomuzki et al. 2010). For instance, there is a trematode that parasitizes certain aquatic snails. Infected snails lose some of their characteristic behavior and will remain on the tops of rocks in streams where food is inadequate and even during peaks of waterfowl activity, making them easy prey for the birds (Levri 1999). Further, parasitism of prey species can indirectly alter the interactions of associated predators, other prey of the predators, and their own prey. When a parasite influences the competitive interaction between two species, it is termed parasite-mediated competition (Figure 8). The parasite can infect one or both of the involved species (Hatcher et al. 2006). For example, the malarial parasite Plasmodium azurophilum differentially infects two lizard species found in the Caribbean, Anolis gingivinius and Anolis wattsi. A. gingivinius is a better competitor than A. wattsi but is susceptible to P. azurophilum, while A. wattsi rarely contracts the parasite. These lizards are found coexisting only when the parasite is present, indicating that the parasite lowers the competitive ability of A. gingivinius' (Schall 1992). In this case, the parasite prevents competitive exclusion, therefore maintaining species diversity in this ecosystem.
Summary
The species interactions discussed above are only some of the known interactions that occur in nature and can be difficult to identify because they can directly or indirectly influence other intra-specific and inter-specific interactions. Additionally, the role of abiotic factors adds complexity to species interactions and how we understand them. That is to say, species interactions are part of the framework that forms the complexity of ecological communities. Species interactions are extremely important in shaping community dynamics. It was originally thought that competition was the driving force of community structure, but it is now understood that all of the interactions discussed in this article, along with their indirect effects and the variation of responses within and between species, define communities and ecosystems (Agrawal 2007).
References and Recommended Reading
Aaron, M. E. & E. J. Farnsworth et al. Facultative Mutualism Between Red Mangroves and Root-Fouling Sponges in Belizean Mangal. Ecology 77, 2431-2444 (1996).
Agrawal, A. A. et al. Filling Key Gaps in Population and Community Ecology. Frontiers in Ecology and the Environment 5, 145-152 (2007).
Brooker, R. W. et al. Don't Diss Integration: A Comment on Ricklefs's Disintegrating Communities. American Naturalist 174, 919-927 (2009).
Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 199 (4335): 1302-1310 (1978).
Deacon, J. in Fungal Biology Vol. 4, 256 (Blackwell Publishing, 2006).
Greenwood, M. J., McIntosh, A.R. & Harding, J. S. Disturbance Across an Ecosystem Boundary Drives Cannibalism Propensity in a Riparian Consumer. Behavioral Ecology 21, 1227-1235.
Gurevitch, J., Scheiner, S. M. & Fox, G. A. The Ecology of Plants Vol. 2, 257 (Sineaur Associates, Inc., 2006).
Hardin, G. The Competitive Exclusion Principle. Science 131,1292-1297 (1960).
Harrison, S. & Cornell, H. Toward a Better Understanding of the Regional Causes of Local Community Richness. Ecology Letters 11, 969-979, (2008).
Hatcher, M. J., Dick, J. T. A. & Dunn, A. M. How parasites affect interactions between competitors and predators. Ecology Letters 9, 1253-1271.
Holomuzki, J. R., Feminella, J. W. & Power, M. E. Biotic Interactions in Freshwater Benthic Habitats. Journal of the North American Benthological Society 29, 220-244.
Horn, H.S. Markovian Properties of Forest Succession. In Cody, M.L., and J.M. Diamond. Ecology and Evolution of Communities. Belknap Press, Mass., U.S., 196-211 (1975).
Huss, M., Van Kooten, T. & Persson, L. Intra-cohort Cannibalism and Size Bimodality: a balance between hatching synchrony and resource feedbacks. Oikos 119, 2000-2011.
Johnson, N. C., Graham, J. H. & Smith, F. A. Functioning of Mycorrhizal Associations along the Mutualism-Parasitism Continuum. New Phytologist 135, 575-586 (1997).
Levri, E. P. Parasite-induced Change in Host Behavior of a Freshwater Snail: parasitic manipulation or byproduct of infection? Behavioral Ecology 10, 234-241 (1999).
Ricklefs, R. E. Disintegration of the Ecological Community. American Naturalist 172, 741-750 (2008).
Roxburgh, S. H., Shea, K. & Wilson, J. B. The Intermediate Disturbance Hypothesis: patch dynamics and mechanisms of species coexistence. Ecology 85, 359-371 (2004).
Schall, J. J. Parasite-mediated Competition in Anolis Lizards. 92, 64-64 (1992).
Volterra, V. Variations and Fluctuations of the Numbers of Individuals in Animal Species Living Together. Reprinted in 1931. In: Chapman, R.N. Animal Ecology. McGraw-Hill, New York (1926).





























