« Prev Next »

A central aim in the discipline of ecology is to determine the underlying causes of variation in the abundance and distribution of species. Variation in population size arises from changes in demographic rates of birth (B), death (D), immigration (I), and emigration (E). Consequently, ecologists study changes in the population size or density (N) at one point in time, t, with some increase in time, t + 1: Nt+1 = Nt + B - D + 1 - E. Ecologists studying population dynamics are concerned with the biological and environmental causes of temporal and spatial changes in population size, including short-term transient dynamics and long-term stability properties.
Fluctuations in population size vary with density-independent and density-dependent factors. For density-independent factors, such as environmental events of weather, demographic rates show no relationship with density. Population dynamics are often shaped by density-dependent processes, whereby demographic rates do exhibit functional relationships with density; such as a reduction in birth rates as resources become increasingly limited. Birth and death rates typically show negative and positive relationships with density, respectively. Examples of density-dependent population dynamics include well-known phenomena of monotonic damping in density toward an equilibrium, as seen in logistic population growth, damped oscillations in which fluctuations in density decrease with time as an equilibrium is approached, and stable limit cycles in which density exhibits repeated oscillations in frequency and amplitude over time. Illustrations of such population dynamic phenomena are thoroughly described in Case (2000).

Interspecific interactions, that is interactions between populations of different species, are an important density-dependent factor shaping population dynamics. Interspecifc interactions occur when the actions, traits, or density of individuals of a population result in a change in an attribute of another species' population. Population attributes include mortality, reproduction, population growth rate, and, among others, population density, all of which are central to the dynamics of a species' population. Arising from the positive (+), neutral (0), and negative (-) effects of one species on a population attribute of another, interspecific interactions are differentiated into six types based on pairwise signs (+, 0, -) of their interaction outcomes: predation (+ -), competition (- -), mutualism (+ +), neutralism (0 0), commensalism (+ 0), and amensalism (0 -).


Advances in understanding the density-dependent population dynamics of predation and competition have been made through the study of their consumer-resource interactions. The consumer-resource interaction is a mechanism for the means by which individuals of different species interact with one another. For instance, predation (+ -) is a direct consumer-resource interaction in which a predator species (consumer) eats a prey species (resource). Exploitative competition (- -) is an indirect consumer-resource interaction in which two competitive species (consumers) exploit a shared nutrient or prey species (resource). The consumer-resource mechanism of interaction relates the process of energy and nutrient transfer between the consumer and the resource, rather than the outcome of the interaction per se.

Central to the consumer-resource interaction is the density-dependent relationship between the per-capita rate of resource exploitation by a consumer and the density or supply of a resource, that is the functional response of the interspecific interaction (Solomon 1949, Holling 1959). Resources are biotic or abiotic factors that increase a demographic rate of the consumer over some range of the supply or abundance of the resource. Consumers deplete the abundance of the resource. In its most general application, a functional response represents the relationship between a demographic rate of one species and resource supply or density of another species. Three types of functional responses are well recognized of species interactions: Type I, Type II, and Type III functional responses, which are linear, hyperbolic and asymptotically saturating, and sigmoidal, respectively. Hyperbolic, saturating functional responses are thought to be most common (Turchin 2003). Illustrations and derivations of functional responses are described in Case (2000).
Theoretical studies of the population dynamics of predation and competition began with the models of Lotka and Volterra in the mid-1920s. Lotka-Volterra models with linear functional responses were largely phenomenological, but they nevertheless provided an initial foundation from which more mechanistic models were later developed. For example, the study of predation was advanced by the formulation of saturating functional responses between consumers and resources through the density-dependent consumption and satiation of predators as a function of prey density (Rosenzweig & MacArthur 1963, Rosenzweig 1969). The study of exploitative competition was advanced by the formulation of the density dependence between competitors and their shared resources, specifically hyperbolic functional responses between a competitor's exploitation and the supply of a resource (Tilman 1982). The consumer-resource interaction, with density-dependent responses of consumers to resources, is central to empirical and theoretical studies of predation and competition.
Initial theory for the population dynamics of mutualism was not developed by Lotka or Volterra, but by Gause and Witt (1935) a decade later. Because mutualism has positive effects on population growth, the negative signs of Lotka-Volterra competition models were replaced by positive signs in developing Lotka-Volterra models of mutualism (Figure 1). Density dependence of the positive mutualistic effects of species on one another is a linear functional response, that is [+ αijNj], where Nj is the density of species j, and αij is the positive per-capita interaction strength of species j on species i (Figure 1). With linear functional responses, increases in the density of one mutualistic species lead to increases in the other and vice versa. As a result, Lotka-Volterra models make unrealistically simple and biologically inaccurate predictions of mutualism: (i) mutualism is unstable, leading to unbounded population growth due to never-ending positive feedback of linear functional responses, and (ii) mutualism is stable only if interaction strengths are weak and/or asymmetric (i.e., αijαji < 1). While weak or asymmetric interaction strengths of Lotka-Volterra models can prevent unbounded growth, they also convey that mutualism has little effect on population dynamics. Due to these theoretical results, mutualism has been (and sometimes continues to be) perceived as an interesting interaction, but one of less importance to ecological dynamics than predation or competition.
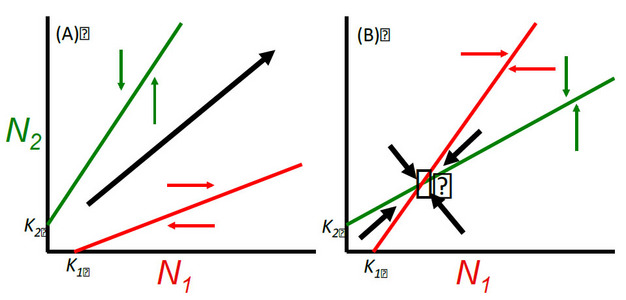
Figure 1 Legend: Consider the basic differential equation for the population dynamics of a single species with logistic population growth, where ri, Ni, and Ki are the intrinsic population growth, population density, and carrying capacity of species i, respectively.
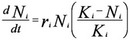
The positive mutualistic effect of species j on species i can be included in this population dynamic equation by adding the term, + αijNj, where αij is the positive per-capita interaction strength of species j on species i. The following set of differential equations represents the positive effects of mutualism on the population dynamics of two mutualistic species:
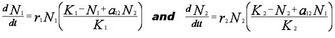
These equations are identical to Lotka-Volterra models of competition, but the negative signs for competition are changed to positive signs for mutualism. In these two dynamical equations, the term + αijNj represents a linear functional response between the effect of the density of Nj on the population growth of Ni. Setting the two equations equal to zero, and solving for N1 and N2, we obtain equations for the zero growth isoclines (dNi/dt = 0) of the interacting species:

These are linear lines with an intercept of Ki and a slope of αij. These two lines can be plotted in (N1, N2)-coordinates, commonly known as phase-plane diagrams. Above the lines Ni decreases and below the lines Ni increases. If the product of the interaction strengths is greater than unity, that is α12α21 > 1, such that the zero growth isoclines of N1 and N2 do not intersect, then the scenario in panel (A) results whereby the interacting populations undergo unbounded population growth. If interaction strengths are weak or asymmetric such that α12α21 < 1, then the isoclines intersect and a stable equilibrium occurs as in panel (B).
The Lotka-Volterra model with linear functional responses provided little theoretical foundation for the population dynamics of mutualism. May (1976) explicitly stated: "Lotka-Volterra models... are inadequate for even a first discussion of mutualism, as they tend to lead to silly solutions in which both populations undergo unbounded exponential growth, in an orgy of mutual benefaction." Gause and Witt (1935) and May (1976) identified that advances over Lotka-Volterra models need to include saturation in the benefits of mutualism. Many studies have examined a wide range of instrinsic and extrinsic factors of pairwise mutualisms that may enhance their stability by limiting such positive feedback, including, for example, intraspecific competition, age structure, spatial structure, interspecific competition, and predation. Compared with predation and competition, however, far less attention has been given to density-dependent functional responses of mutualistic interactions that may contribute to their population dynamics.
What are the appropriate density-dependent functional responses for mutualistic interactions? Can density-dependent functional responses be a form of saturation in the benefits of mutualism that resolves the shortcomings of Lotka-Volterra models? In general, mutualism can be expected to have nonlinear functional responses for which the demographic rates of one mutualistic species vary with the density of another mutualistic species (Holland et al. 2002, Thompson et al. 2006, Holland & DeAngelis 2010). For example, as pollinator abundance increases, more flowers are pollinated, but at some point pollinator density is sufficiently large that all flowers are pollinated and further increases in pollinator density do not increase plant reproduction. As with predator consumption of prey, pollinator consumption of floral nectar increases with nectar supply, but at some point pollinators become satiated or otherwise unable to handle further increases in nectar supply rates.
Models with asymptotically saturating functional responses for the benefits of mutualism lead to population dynamics that differ from Lotka-Volterra models (Figure 2). With saturating functional responses, zero growth isoclines are curvilinear, rather than linear as in Lotka-Volterra models, resulting in a stable equilbrium not conditional upon interaction strengths being weak or asymmetric (i.e., αijαji < 1). Mutualism no longer leads to unbounded population growth. Mutualism increases equilibrium densities of the interacting species above their densities at carrying capacity in isolation of interactions with one another. Population dynamic models with saturating functional responses are an advance over and help to resolve shortcomings of Lotka-Volterra theory for mutualism.
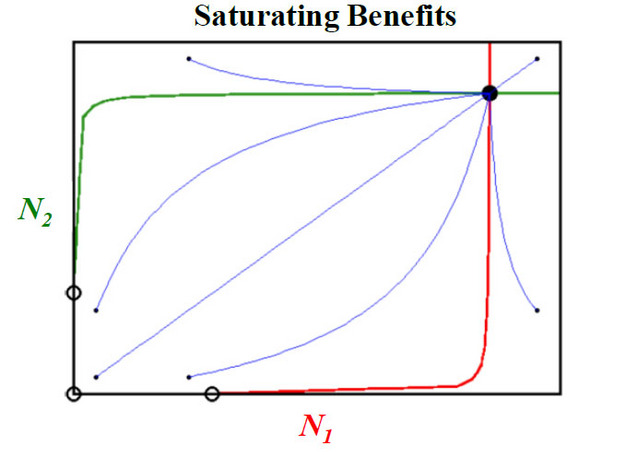
Figure 2 Legend: Consider a basic differential equation for the population dynamics of a single species with logistic population growth, where ri, Ni, and di are the intrinsic population growth, population density, and mortality rate of species i, respectively. In this case, self-limitation is represented by -diNi2 rather than K-N/K as in the Lotka-Volterra model.
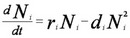
Instead of a linear functional response (αijNj) as in the Lotka-Volterra model, the positive saturating benefits of species j on species i is represented by including a separate term for the nonlinear hyperbolic functional response, [αij Nj / (bj + Nj)], where αij is the positive interaction strength of species j on species i and bj is the half-saturation constant of the Michaelis-Menten function (most any such saturating function produces similar effects). The following set of differential equations represents the population dynamics of two mutualistic species with the saturating positive effects of mutualism:

Setting the two equations equal to zero, and solving for N1 and N2, we obtain equations for the zero growth isoclines (dNi/dt = 0) of the interacting species:
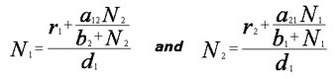
The curvilinear zero growth isoclines, with intercepts of ri/di, are plotted in red and green for N1 and N2, respectively. The intercepts are the equilibrium points of each species in the absence of mutualistic interactions, as depicted by the two open circles on the N1 and N2 axes of the phase-plane diagram. Starting with the origin and moving clock-wise, the equilibrium points are unstable, saddle point, stable equilibrium, saddle point. Transient dynamics of some population trajectories (blue lines) in phase-plane space are presented with initial conditions for N1 and N2 of each trajectory depicted with filled black circles.
Models with saturating functional responses are a minimal starting point for theoretical analyses of the population dynamics of mutualism, but they (like most models) have inconsistencies with empirical knowledge. All transient dynamics lead to the stable equilibrium of mutualism (Figure 2). The model does not make any predictions of dynamics that include population fluctuations, damped oscillations, limit cycles or any population dynamic phenomena beyond monotonic damping in density toward a stable equilibrium. Yet, in nature mutualistic populations can fluctuate in space and time, and mutualistic species may overexploit and potentially drive one another to extinction. While population dynamic models with saturating functional responses for the benefits of mutualism represent a key advance over Lotka-Volterra models, they still do not make some basic predictions of phenomena known to occur in nature.
Recognition that mutualism, like predation and competition, is based on the consumer-resource mechanism of interspecific interaction, provides further insights into their population dynamics (Figure 3), including predictions of empirical phenomena that occur in nature (Holland & DeAngelis 2010). Nearly all pairwise mutualisms can be described as uni-directional or bi-directional consumer-resource interactions. In uni-directional consumer-resource mutualisms, one species functions as a consumer and the other as a resource, but neither functions as both. Resources produced by a mutualistic species attract and reward a consumer, which in the process of exploiting the resource provisions the former with a service of dispersal or defense. For example, plants produce nectar resources that are consumed by animals which disperse pollen and pollinate flowers. Similarly, plants produce extrafloral nectar resources that are consumed by ants which defend plants against their natural enemies. In bi-directional consumer-resource mutualisms, each species functions as both a consumer and a resource of the other. Each species exploits the other as a resource and each also supplies the other with a resource. Common examples include carbon and nutrient exchanges between plants and mycorrhizal fungi, and between plants and rhizobial bacteria. Indirect mutualisms can arise when the effects of the two indirect mutualistic species on one another are mediated entirely by the density or traits of a third species that is a consumer or resource of one or both of the indirect mutualistic species, such as interactions between cleaner and client fishes.
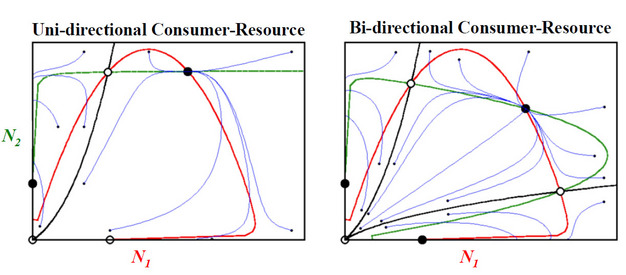
Figure 3 Legend: Holland and DeAngelis (2010) provide details on, and the theoretical analyses of, consumer-resource models of mutualistic interactions. Here, the emphasis is on the concepts and insights gained from recognizing mutualisms as consumer-resource interactions, rather than the more complicated models and analyses. These two phase-plane diagrams are analyses of the short-term transient dynamics and long-term stabililty properties of mutualistic interactions between two facultative species that exhibit uni-directional consumer-resource interactions and bi-dirctional consumer-resource interactions, with population densities of N1 and N2, respectively. Red and green lines are zero-growth isoclines (dNi/dt = 0) for N1 and N2, respectively. Stable and unstable equilibrium points are identified by filled and open circles, respectively. Saddle points have a black line (separatrix) passing through them to the origin, subdividing phase-plane space into different basins of attraction that correspond with a particular equilibrium point. Starting with the origin and moving clock-wise, the equilibria for uni-directional consumer-resource mutualism include: unstable equilibrium, stable equilibrium, saddle point, stable equilibrium, saddle point. For bi-directional consumer-resource mutualisms, the equilibrium points include: unstable equilibrium, stable equilibrium, saddle point, stable equilibrium, saddle point, stable equilibrium. Transient dynamics of some population trajectories (blue lines) in phase-plane space are presented, for which the initial conditions for N1 and N2 of each trajectory are depicted with filled black circles.
With the consumer-resource mechanism incorporated into models of mutualism, population dynamic phenomena well recognized to occur in nature are in turn predicted by theory of mutualism. As exemplified by transient dynamics and stability properties of uni- and bi-directional consumer-resource mutualisms (Figure 3), such phenomena are now predicted to include not only monotonic damping, but also damped oscillations toward an equilibrium and stable limit cycles. Also, density-dependent overexploitation may occur in which one mutualistic species leads to the extinction of another. In sum, by unifying predation, competition, and mutualism under the common ecological framework of consumer-resource theory, we may also gain a better understanding of the universal features of interspecific interactions in general.
References and Recommended Reading
Boucher, D. H., James, S. & Keeler, K. H. The ecology of mutualisms. Annual Review of Ecology and Systematics 13, 315 -347 (1982).
Boucher, D. H. The Biology of Mutualism: Ecology and Evolution. New York, NY: Oxford University Press, 1985.
Bronstein, J. L. "Mutualisms," in Evolutionary Ecology: Concepts and Case Studies, eds. C. W. Fox, D. A. Roff, & D. J. Fairbairn (New York, NY: Oxford University Press, 2001) 315-330.
Bronstein, J. L. "Mutualism and symbiosis," in The Princeton Guide to Ecology, ed. S. A. Levin (Princeton, NJ: Princeton University Press, 2009) 233-238.
Case, T. J. An Illustrated Guide to Theoretical Ecology. New York, NY: Oxford University Press, 2000.
Gause, G. F. & Witt, A. A. Behavior of mixed populations and the problem of natural selection. The American Naturalist 69, 596-609 (1935).
Holland, J. N., DeAngelis, D. L. & Bronstein, J. L. Population dynamics and mutualism: Functional responses of benefits and costs. The American Naturalist 159, 231-244 (2002).
Holland, J. N. & Bronstein, J. L. 2008. "Mutualism," in Population Dynamics, Encyclopedia of Ecology, Vol. 3, eds. S. E. Jorgensen & B. D. Fath (Oxford, UK: Elsevier, 2008) 2485-2491.
Holland, J. N. & DeAngelis, D. L. A consumer-resource approach to the density-dependent population dynamics of mutualism. Ecology 91, 1286-1295 (2010).
Holling, C. S. The components of predation as revealed by a study of small mammal predation of the European pine sawfly. Canadian Entomologist 91, 293-320 (1959).
MacArthur, R. H. Geographical Ecology. New York, NY: Harper and Row, 1972.
May, R. M. 1976. "Models of two interacting populations," in Theoretical Ecology: Principles and Application, ed. R. M. May (Philadelphia, PA: Saunders, 1976) 78-104.
Murdoch, W. M., Briggs, C. J. & Nisbet, R. M. Consumer-Resource Dynamics. Princeton, NJ: Princeton University Press, 2003.
Rosenzweig, M. L. & MacArthur, R. H. Graphical representation and stability conditions of predator-prey interactions. The American Naturalist 97, 209-223 (1963).
Schwartz, M. W. & Hoeksema, J. D. Specialization and resource trade: Biological markets as a model of mutualisms. Ecology 79, 1029-1038 (1998).
Solomon, M. E. The natural control of animal populations. Journal of Animal Ecology 18, 1-35 (1949).
Thompson, A. R., Nisbet, R. M. & Schmitt, R. J. Dynamics of mutualist populations that are demographically open. Journal of Animal Ecology 75, 1239-1251 (2006).
Tilman, D. Resource Competition and Community Structure. Princeton, NJ: Princeton University Press, 1982.
Turchin, P. Complex Population Dynamics: A Theoretical/Empirical Synthesis. Princeton, NJ: Princeton University Press, 2003.






























