« Prev Next »
Lakes and ponds (also known as lentic systems) are a diverse set of inland freshwater habitats that exist across the globe and provide essential resources and habitats for both terrestrial and aquatic organisms. Although widely distributed and vital for many species, including humans, these habitats account for just over 3% of the Earth's surface (Downing et al. 2006). Importantly, these rare systems are increasingly threatened by the human demand for freshwater as well as human activity (Dudgeon et al. 2006). Recent evidence suggests that freshwater systems are more imperiled than marine and terrestrial ones (Sala et al. 2000, Dudgeon et al. 2006). Effective conservation and management of these systems relies on our ability to fulfill the growing human demand for freshwater while maintaining system integrity.The study of lentic systems is conducted within the field of limnology. Limnology is the study of the structural and functional interrelationships of organisms of inland waters as they are affected by their dynamic physical, chemical, and biotic environments (Wetzel 2001). Several excellent books published within the past decade provide in-depth coverage of lakes and ponds (Wetzel 2001, Brönmark & Hansson 2005, Moss 2010). Another excellent resource is the Encyclopedia of Inland Waters (2009). In this article, we present a brief overview of lentic systems and refer the reader to specific literature for more detailed information.
Physical and Chemical Structure
Size, formation, and succession
Lentic systems vary from ephemeral ponds that fill briefly during rain events and then dry out, to permanent water bodies such as lakes (Figure 1). The world's largest freshwater lake, based on area, is Lake Superior of North America (82,100 km2) while the deepest is Baikal Lake of Asia (max depth = 1741 m; mean depth 730 m). Lakes and ponds are formed by a diversity of processes. For example, the geologic uplift of mountains can lead to depressions that fill with water to create lakes. The subsidence of land along faults between tectonic plates has lead to the formation of deep rift lakes such as the Rift Valley Lakes of eastern Africa. Lakes can also form in the crater of an inactive volcano. The movement of glaciers has been an important force in creating lakes and ponds. For example, the Laurentian Great Lakes were created following the retreat of the Laurentide ice sheet during the last ice age 10,000 years ago. Rivers also play a role in creating lakes. For example, a wide meander in a river can be cut off from the main river to form an oxbow lake. Additionally, the flood plain of a river can inundate surrounding habitats creating small ponds as floodwaters recede.
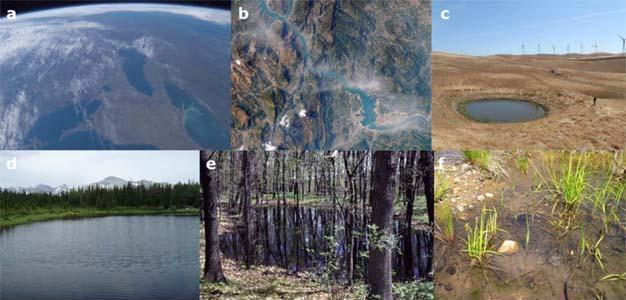
Humans also create lentic habitats. Across the world, humans have constructed impoundments and reservoirs to provide water resources for humans and livestock, generate energy, or control flooding. While large water bodies have historically received most of the attention, ponds are the numerically dominant lentic habitat (Downing et al. 2006). Ponds are generally <2 hectares in size, shallow (<3 m), and dominated by aquatic plants. Ponds have a diversity of ecological and geological origins including man-made impoundments. Moreover, due to their small size, ponds often have characteristics that are distinct from larger water bodies, which we will point out below.
Once formed, lentic systems can undergo successional development in which the water body is gradually transformed and incorporated into the terrestrial landscape. Successional development is driven by sedimentation of inorganic and organic matter into the system. Sedimentation rates are influenced by the size and depth of the system, the surrounding terrestrial landscape, climate, productivity, and decomposition (Wetzel 2001). Shallow ponds provide an excellent example of successional development. As sedimentation occurs, the depth of the pond is reduced which allows vegetation to gradually encroach into the system. Over time, this leads to swamp conditions with standing water present among the vegetation. As sedimentation continues, marsh conditions are established with no standing water but water saturated sediments. The terminal stage of succession can include predominately dry land or bogs depending on such factors as the location of the water table and climatic conditions.
Thermal stratification
Light transmission
Nutrient inputs and cycling
Biotic Structure: Lake Zones and Food Webs
Nearly 6% of described species (i.e., 100,000 species) are supported by freshwater systems (Dudgeon et al. 2006). Moreover, some of the world's oldest lakes (>1 million years old) contain a high diversity of endemic species that have evolved through the process of adaptive radiation (Cristescu et al. 2010). In lakes and ponds, much of the species diversity is concentrated in the littoral zone, near the shore, where algae and plants thrive in the abundant light needed for photosynthesis. Living within the plant matter is a cornucopia of animals including snails, amphibians, crustaceans, insects, and fish. Beyond the littoral zone is the limnetic zone. This is the zone of open water where light is still able to penetrate and support photosynthetic algae (i.e., photic zone). Consumers in this zone include zooplankton, which feed on the algae, some insects, and fish. Finally, the benthic zone is the bottom sediment (e.g., mud, sand, rock) of the habitat, which is dominated by invertebrate species.
Primary producers
Primary producers are broadly divided into three groups, periphyton, phytoplankton, and macrophytes. Periphyton grows on substrates such as mud, sand, rocks, and leaf litter where it appears as a green or brown film. This is an important resource for a diversity of herbivores including macroinvertebrates (e.g., snails) and amphibian larvae. Phytoplankton is algae suspended in the water column. Phytoplankton is divided into several groups (e.g., cyanobacteria, diatoms, chrysophytes, dinoflagellates, desmids) that tend to dominate at different levels of lake productivity. Herbivores such as zooplankton are common consumers of phytoplankton. Macrophytes are generally the most visible group of primary producers in lakes and ponds (Figure 2). Macrophyte species possess a diversity of growth forms. Emergent macrophytes undergo photosynthesis above the water and are typically found close to the shoreline. As depth increases, submerged macrophytes that undergo photosynthesis under the water, dominate. Macrophytes provide resources for many herbivores as well as important refuge from predators.

Consumers
Patterns in Species Richness
Species-area relationships have a long history in ecological research and work on lentic systems has widely supported an increase in species richness with increasing habitat area (MacArthur & Wilson 1967, Browne 1981, Wellborn et al. 1996, Dodson et al. 2000). However, there are many other biotic and abiotic factors that change alongside increases in habitat area, such as permanence, water acidity, and predation pressure. All of these may play important roles in influencing species richness and community structure (Wellborn et al. 1996, Brönmark & Hansson 1998, Wetzel 2000, Williams 2001). Physical environmental factors such as hydroperiod and water chemistry can limit the breadth of species distributions due to physiological constraints (Williams 2001). For example, species that are tolerant of pond drying inhabit a diversity of habitat types (i.e., lakes and ephemeral ponds) whereas species that are intolerant of pond drying are restricted to lakes (Wellborn et al. 1996, Williams 2001). As a consequence of these physiological constraints, species richness tends to increase as hydroperiod increases. However, biotic interactions also can play an important role in structuring communities. Factors such as competition and predation can function to limit the abundance of a species (Kerfoot & Sih 1987, Brönmark & Hansson 2002). For example, many species tolerant of pond drying are frequently outcompeted in permanent waters. Hence their abundance is low or they mainly occur in marginal habitat of the shores, which largely functions as a temporary habitat. Additionally, the transition to more permanent water is often associated with an increase in the abundance of predators (Wellborn et al. 1996). For example, fish play an important role in reducing the abundance of species and influencing species composition and richness in permanent lakes. Thus, abiotic and biotic factors can interact to influence species richness and community structure in lentic systems.Threats to Lentic Ecosystems
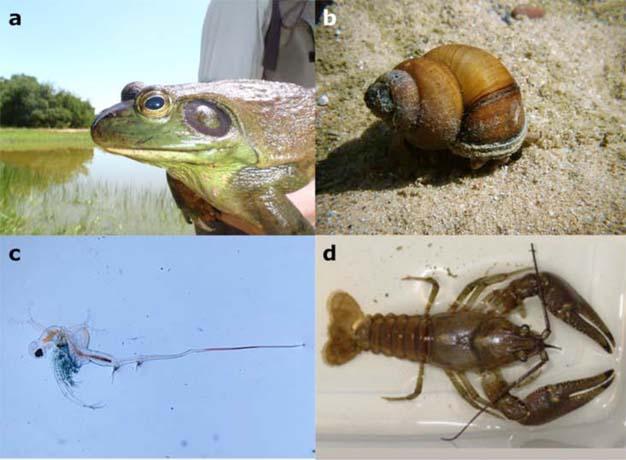
The biodiversity of freshwater habitats is increasingly threatened by human activities. Habitat loss, eutrophication, acidification, chemical contamination, global warming, and exotic species are just some of the factors that have directly or indirectly impacted lentic systems (Brönmark & Hansson 2002). Substantial progress has been made in identifying how some of these threats affect aquatic communities, and how to develop management strategies to reduce or eliminate their impacts. For example, the input of phosphorus, a limiting nutrient in lentic systems, was identified as a major contributor to lake eutrophication (Schindler 1974). Consequently, laws have been enacted to treat wastewater and reduce sewage input of phosphorus into lentic systems. Although some lentic systems have recovered, eutrophication remains a persistent problem due to agricultural intensification and other non-point pollution sources. Another challenging problem is the introduction of exotic species including plants, animals, and pathogens into lentic systems (Figure 3). In North America, for example, exotic wetland plants (e.g., purple loosestrife [Lythrum salicaria], Eurasian watermilfoil [Myriophyllum spicatum]) and animals (e.g., grass carp [Ctenopharyngodon idella], zebra mussel [Dreissena polymorpha]) can outcompete native species for nutrients or space. The introduction of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) into Central America is implicated in the extinction of numerous amphibian species (Kilpatrick et al. 2010). While the potential negative consequences of exotic species introduction are well recognized, controlling their spread is difficult and, once established, eliminating them from communities has been difficult. Lastly, human-mediated habitat loss and alteration are increasing concerns driven by the expanding need for agricultural lands as well as development. These activities not only lead to the loss of habitat for species and vital water resources for a region, but also fragment and alter the landscape in which the remaining habitats are embedded. The combination of these threats and the scarcity of freshwater habitats make lentic systems one of the most imperiled on the planet (Sala et al. 2000, Dudgeon et al. 2006).
Acknowledgements
Glossary
Adaptive radiation: The evolution of ecological and phenotypic diversity within a rapidly multiplying lineage
Aphotic zone: Depth of the water where there is little to no sunlight for photosynthesis to occur
Benthic zone: The sediment layer of lentic systems
Epilimnion: The top layer of water in a thermally stratified water body
Eutrophic: A lentic system with high nutrient concentrations and high plant growth
Food web: Feeding relationships among organisms within an ecosystem or community
Hydroperiod: The length of time that a lentic system contains standing water
Hypolimnion: The bottom layer of water in a thermally stratified water body
Hysteresis: A system that exhibits path dependence such that the output depends in part on the internal state of system and not only on its input
Inverse stratification: Minor density gradient that develops between surface waters at 0°C (less dense) and lower waters at 4°C (more dense) leading to weak stratification
Laurentian Great Lakes: The five largest North American lakes formed by the retreat of the Laurentide ice sheet about 10,000 years ago
Limnetic zone: The open-water habitat of lentic systems beyond the littoral zone
Limnology: The study of the structural and functional interrelationships of organisms of inland waters as they are affected by their dynamic physical, chemical, and biotic environments
Littoral zone: The near-shore habitat of lentic systems
Macrophyte: An aquatic plant that grows in or near water and is either emergent, submergent, or floating
Oligotrophic: A lentic system with low nutrient concentrations and low plant growth
Oxbow lake: Water body formed when a wide meander from a river system is cut off
Periphyton: Complex mixture of algae, cyanobacteria, heterotrophic microbes, and detritus that are attached to submerged surfaces in aquatic environments
Photic zone: Depth of the water that is exposed to sufficient sunlight for photosynthesis to occur
Phytoplankton: Photosynthesizing microscopic organisms that are suspended in the photic zone of the water column
Reciprocal subsidies: Energy flux among terrestrial and aquatic habitats
Successional development: Temporal changes in the composition or structure of an ecological community
Thermal stratification: The temperature gradient observed at different depths in a water body due to the change in water's density with temperature
Thermocline: The distinct layer in a thermally stratified water body in which temperature changes more rapidly with depth than it does in the epilimnion or hypolimnion
References and Recommended Reading
Brönmark, C. & Hansson, L. A. Environmental issues in lakes and ponds: Current state and perspectives. Environmental Conservation 29, 290-307 (2002).
Brönmark, C. & Hansson, L. A. The Biology of Lakes and Ponds, 2nd ed. Oxford, UK: Oxford University Press, 2005.
Browne, R. A. Lakes as islands: Biogeographic distribution, turnover rates, and species composition in the lakes of central New York. Journal of Biogeography 8, 75-83 (1981).
Cristescu, M. E. et al. Ancient lakes revisited: From the ecology to the genetics of speciation. Molecular Ecology 19, 4837-4851 (2010).
Dodson, S. I., Arnott, S. E. & Cottingham, K. L. The relationship in lake communities between primary productivity and species richness. Ecology 81, 2662-2679 (2000).
Downing, J. A. et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnology and Oceanography 51, 2388-2397 (2006).
Dudgeon, D. et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biological Reviews 81, 163-182 (2006).
Elser, J. J. et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10, 1135-1142 (2007).
Kerfoot, W. C. & Sih, A. Predation: Direct and Indirect Impacts on Aquatic Communities. Lebanon, NH: University Press of New England, 1987.
Kilpatrick, A. M., Briggs, C. J. & Daszak, P. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends in Ecology & Evolution 25, 109-118 (2010).
Lamberti, G. A., Chaloner, D. T. & Hershey, A. E. Linkages among aquatic ecosystems. Journal of the North American Benthological Society 29, 245-263 (2010).
Likens, G. Encyclopedia of Inland Waters. Academic Press, 2009.
Moss, B. Ecology of Fresh Waters: A View for the Twenty-First Century, 4th ed. Wiley-Blackwell Publishing, 2010.
Nakano, S. & Murakami, M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences of the United States of America 98, 166-170 (2001).
Sala, O. E. et al. Biodiversity - Global biodiversity scenarios for the year 2100. Science 287, 1770-1774 (2000).
Scheffer, M. & van Nes, E. H. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584, 455-466 (2007).
Schindler, D. W. Eutrophication and recovery in experimental lakes - Implications for lake management. Science 184, 897-899 (1974).
Wellborn, G. A., Skelly, D. K. & Werner, E. E. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27, 337-363 (1996).
Wetzel, R. Limnology: Lake and River Ecosystems, 3rd ed. Academic Press, 2001.
Wetzel, R. & Likens, G. Limnological Analyses, 3rd ed. New York, NY: Springer-Verlag, 2000.
Williams, D. The Ecology of Temporary Waters. Caldwell, NJ: Blackburn Press, 2001.






























