« Prev Next »
Introduction

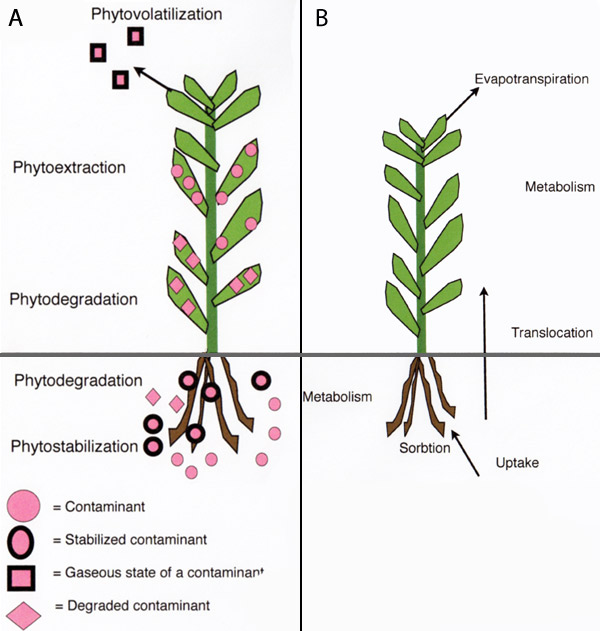
Phytoremediation basically refers to the use of plants and associated soil microbes to reduce the concentrations or toxic effects of contaminants in the environment. Phytoremediation is widely accepted as a cost-effective environmental restoration technology. Phytoremediation is an alternative to engineering procedures that are usually more destructive to the soil. Phytoremediation of contaminated sites should ideally not exceed one decade to reach acceptable levels of contaminants in the environment. Phytoremediation is, however, limited to the root-zone of plants. Also, this technology has limited application where the concentrations of contaminants are toxic to plants. Phytoremediation technologies are available for various environments and types of contaminants. These involve different processes such as in situ stabilization or degradation and removal (i.e., volatilization or extraction) of contaminants (Figure 3A; Table 1).
| Technology |
Action on Contaminants
|
Main Type of Contaminants
|
Vegetation |
| Phytostabilization |
Retained in situ
|
Organics and metals |
Cover maintained |
| Phytodegradation |
Attenuated in situ
|
Organics |
Cover maintained |
| Phytovolatilization | Removed |
Organics and metals |
Cover maintained |
| Phytoextraction | Removed | Metals |
Harvested repeatedly |
|
Table 1: Comparison between phytoremediation technologies
| |||
Phytostabilization

Phytodegradation

Phytovolatilization
Phytovolatilization involves the uptake of contaminants by plant roots and its conversion to a gaseous state, and release into the atmosphere. This process is driven by the evapotranspiration of plants (Figure 3B). Plants that have high evapotranspiration rate are sought after in phytovolatilization. Organic contaminants, especially volatile organic compounds (VOCs) are passively volatilized by plants. For example, hybrid poplar trees have been used to volatilize trichloroethylene (TCE) by converting it to chlorinated acetates and CO2. Metals such as Se can be volatilized by plants through conversion into dimethylselenide [Se(CH3)2]. Genetic engineering has been used to allow plants to volatilize specific contaminants. For example, the ability of the tuliptree (Liriodendron tulipifera) to volatilize methyl-Hg from the soil into the atmosphere (as Hg0) was improved by inserting genes of modified E. coli that encode the enzyme mercuric ion reductase (merA).
Phytoextraction
Phytoextraction uses the ability of plants to accumulate contaminants in the aboveground, harvestable biomass (Figure 3B). This process involves repeated harvesting of the biomass in order to lower the concentration of contaminants in the soil. Phytoextraction is either a continuous process (using metal hyperaccumulating plants, or fast growing plants), or an induced process (using chemicals to increase the bioavailability of metals in the soil). Continuous phytoextraction is based on the ability of certain plants to gradually accumulate contaminants (mainly metals) into their biomass. Certain plants can hyperaccumulate metals without any toxic effects. These plants are adapted to naturally occurring, metalliferous soils. More than 400 plant species can hyperaccumulate various metals. However, most plants can only hyperaccumulate one specific metal.
Hyperaccumulating plants can contain more than 1% of a metal in their dry biomass. For example, the hyperaccumulating plant Berkheya coddii was found to contain as much as 3.8% of Ni in the dry, aboveground biomass, when grown in contaminated soil. It is possible to extract metals from the harvested biomass in a process termed phytomining. The underlying mechanism of hyperaccumulation of metals in plants is the overexpression of genes that regulate cell membrane transporters. These include the Cu-transporter (COPT1) and Zn-transporter (ZNT1). The main limitations on the use of hyperaccumulating plants in phytoextraction are slow growth and low biomass production. The effectiveness of phytoextraction is a function of a plant's biomass production and the content of contaminants in the harvested biomass. Therefore, fast-growing crops that accumulate metals have a great potential in phytoextraction. The use of crops in phytoextraction can be improved by manipulation of their associated soil microbes. Inoculation of plant growth promoting bacteria (PGPR) and AMF can increase plant biomass. The AMF-plant symbiosis usually results in reduced accumulation of metals in the aboveground biomass of plants. Therefore, suppressing AMF activity, by using specific soil fungicides, has resulted in increased metal accumulation in plants. The role of AMF in regulating metal uptake by plants appears to vary depending on numerous factors, such as AMF populations, plant species, nutrient availability, and metal content in the soil. Also, this regulation of AMF is usually metal-specific; where the uptake of essential metals is generally increased, but the uptake of non-essential metals is inhibited. However, exceptions have been found where AMF increases uptake of Ni, Pb, and As in plants. Induced phytoextraction involves the use of fast-growing crops and chemical manipulation of the soil. Low bioavailability of metals in the soil is a limiting factor in phytoextraction. The bioavailability of metals can be increased by the use of synthetic chelates such as ethylenediaminetetracetic acid (EDTA) or acidifying chemicals (e.g., NH4SO4). The use of synthetic chelates increases the absorption of metals to the root and the translocation of metals from the roots to the foliage (Figure 3). The timing of chelate application is critical, and should ideally take place at the peak of biomass production. The effectiveness of using EDTA was demonstrated by growing corn (Zea mays) in Pb-contaminated soil treated with 10 mmol kg-1 EDTA. This resulted in a high accumulation of Pb (1.6% of shoot dry weight), and facilitated the translocation of Pb from the roots to the foliage. Some drawbacks of using synthetic chelates in phytoremediation are the result of increased solubility of the metals within the soil. In turn this increases the risk of metal migration through the soil profile and into the groundwater. However, a possible solution is to treat contaminated soil ex situ in a confined site with an impervious surface. Also, periodic application of low doses of synthetic chelates reduces the risk of metal migration.
Summary
Contaminants in the environment pose a global problem for wildlife and human health. Phytoremediation is a recently developed technology that offers a cost-effective solution by using plants, and associated soil microbes, to reduce the content, or toxic effects, of contaminants in the environment. Phytoremediation technologies include: (a) phytostabilization, where contaminants are retained in the soil, (b) phytodegradation, where organic contaminants are converted to less harmful substances, (c) phytovolatilization, where contaminants are converted inside plants to a gaseous state and released into the atmosphere via the evapotranspiration process, and, (d) phytoextraction, where plants are used to accumulate contaminants in the aboveground, harvestable biomass.
References and Recommended Reading
Assunção, A. G. L. et al. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell and Environment 24, 217–226 (2001) doi:10.1111/j.1365-3040.2001.00666.x.
Bizily, S. P., Rugh, C. L., & Meagher, R. B. Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nature Biotechnology 18, 213–217 (2000) doi:10.1038/72678.
Boyajian, G. E. & Carreira, L. H. Phytoremediation: A clean transition from laboratory to marketplace. Nature Biotechnology 15, 127–128 (1997) doi:10.1038/nbt0297-127.
Clemens, S., Palmgren, M. P., & Krämer, U. A long way ahead: Understanding and engineering plant metal accumulation. Trends in Plant Science 7, 309–315 (2002) doi:10.1016/S1360-1385(02)02295-1.
Gohre, V. & Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 223, 1115–1122 (2006) doi:10.1007/s00425-006-0225-0.
Jaffre, T. et al. Sebertia accuminata: A nickel accumulating plant from New Caledonia. Science 193, 579–580 (1976) doi:10.1126/science.193.4253.579.
Lanphear, B. P. et al. Low-level environmental lead exposure and children's intellectual function: An international pooled analysis. Environmental Health Perspectives 113, 894–899 (2005) doi:10.1289/ehp.7688.
Lasat, M. M. Phytoextraction of toxic metals: A review of biological mechanisms. Journal of Environmental Quality 31, 109–120 (2002) doi:10.2134/jeq2002.0109.
Meers, E. et al. Chemically assisted phytoextraction: A review of potentially soil amendments for increasing plant uptake of heavy metals. International Journal of Phytoremediation 10, 390–414 (2008) doi:10.1080/15226510802100515.
Pilon-Smits, E. Phytoremediation. Annual Review of Plant Biology 56, 15–39 (2005).
Rugh, C. L. et al. Development of transgenic yellow poplar for mercury phytoremediation. Nature Biotechnology 16, 925–928 (1998) doi:10.1038/nbt1098-925.
Salt, D. E., Smith, R. D., & Raskin, I. Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology 49, 643–668 (1998).
Vangronsveld, J. et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environmental Science and Pollution Research 16, 765–794 (2009) doi:10.10070/s11356-009-0213-6.
Wu, C. H. et al. Engineering plant-microbe symbiosis for rhizoremediation of heavy metals. Applied and Environmental Microbiology 72, 1129–1134 (2006) doi:10.1128/AEM.72.2.1129-1134.2006.































