« Prev Next »
|
Sensory modality
|
Example senses
|
| Vibration | Hearing, vibration detection |
|
Body movement |
Equilibrium (balance) |
|
Physical contact |
Touch |
| Chemicals |
Smell, taste, pheromone detectors |
|
Electromagnetic energy |
Vision, electric field detection, magnetic field detection |
| Temperature | Heat "vision," temperature detection |
| Table 1: Some examples of different sensory modalities. For each modality, some examples of sensory systems that use that modality are provided. | |
Animal sensory ecology allows us to peer into the perceptual world that animals inhabit, which gives us a better understanding of animal behavior. It can also give us new ways of "looking" at the world, especially when we can record and analyze stimuli that are in the ultra or infra regions, such as the canine ability to detect odors, which is at least 1,000,000 times more sensitive than a human's (Olender et al. 2004), or stimuli that are utterly impossible for humans to detect because we lack the sensory organs to do so, such as the ability to detect electric fields (Camperi et al. 2007, Heiligenberg 1991), or magnetic fields (Akesson & Backman 1999, Holland et al. 2010, Weindler et al. 1995). In order to study stimuli that we cannot perceive, we must develop devices that can detect them for us, such as cameras that detect light that our eyes cannot see, or microphones that can record sounds that we cannot hear. In this article we will limit our discussion to vision and hearing, which are the most highly developed human senses. We also limit ourselves primarily to vertebrate organisms, but this should not be taken to mean that other animals do not have similar sensory systems or that they are not of interest — we are only starting with the most basic background on animal sensory systems, and much more is available if you choose to explore this topic further.
Vision
The sense of vision depends on the ability of photoreceptors to detect the presence of specific types of electromagnetic energy (Figure 1). We describe electromagnetic energy based on its wavelength (or its frequency), and the wavelengths that humans can see form the familiar colors of the rainbow. These colors correspond to wavelengths of roughly 700 nm (red) down to about 400 nm (violet). For wavelengths just beyond the range that human eyes can see, we use the terms infrared (IR; wavelengths between ~1000 nm and 1 mm) and ultraviolet (UV; roughly 100-400 nm) (Ryer 1997). The IR and UV spectra are well within the range that many animals can see. While your eyes cannot detect it, infrared radiation is familiar to you as the type of warmth you feel when you put your hand near a source of heat, such as a stove. Any object that is warmer than its environment is emitting infrared radiation, and some animals have receptors that can detect this as a type of "heat vision" (Newman & Harline 1982, Sichert et al. 2006). This ability is highly beneficial to predators that feed on birds and mammals, because their prey are almost always warmer than their environment. For example snakes, such as pit vipers, pythons, and boa constrictors, have special pits on their heads that contain IR-sensitive receptors (Figure 2; Newman & Hartline 1982, Pappas et al. 2004). These pits allow this predator to hide in a secluded location and wait until an IR-emitting prey strays within range of a strike (Newman & Hartline 1982, Ebert et al. 2007). These receptors also may enable the snake to detect and avoid mammalian predators, or to find locations that are the appropriate temperature for sleeping or basking (Ebert et al. 2007, van Dyke & Grace 2010).
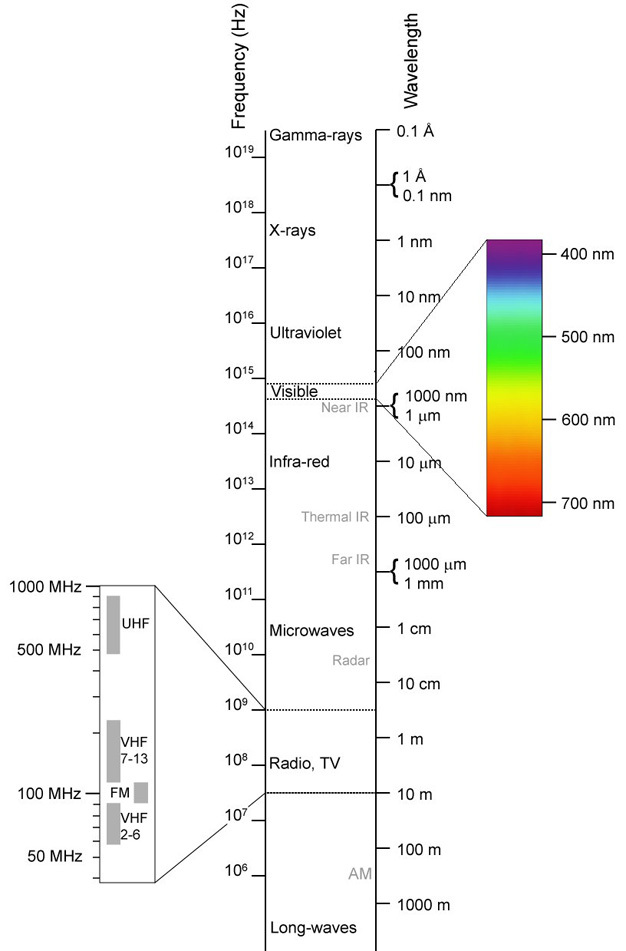

There are also animals that can see ultraviolet radiation, including birds (Rubene et al. 2010), butterflies (Rutowski et al. 2007), and bees (Arnold et al. 2010). Some of the best studied examples of UV vision are of bees and the plants that they pollinate. Many of these plants have flowers that reflect light in both the visible and the UV range (Arnold et al. 2010, Dusenbery 1992). When those flowers are examined in UV light, patterns of light and dark are visible. These patterns guide bees to the part of the flower where they will find nectar (Figure 3). These patterned calling cards also benefit the plant by directing a bee to portions of the flower where it will pick up pollen that can then be spread to other flowers. While bees can also see quite well in the visible spectrum (Dusenbery 1992, Kevan et al. 2001), the addition of UV light gives them access to information that would be lacking if they were using eyes with only human sensitivity. It is notable that in locations where bees do not pollinate flowers regularly, the number of plant species with UV-reflecting flowers is significantly lower than in areas where bees are important pollinators (Kevan et al. 2001).

Hearing
Many animals can detect the presence of vibrations in the environment around them using a variety of vibration-detecting structures. This can include vibrations spread through a substrate, such as the ones produced by an animal walking on a plant (Caldwell et al. 2010, Cocroft & Rodriguez 2005), or vibrations in the air or water around them (Stebbins 1983). Your ability to detect vibrations in the air is referred to as your sense of hearing. Vibrations of the air cause a series of structures inside your ear to vibrate, leading to the production of electrical signals in nerves that connect to your brain. Your brain then interprets these nerve signals as a sound. When measuring vibrations we typically refer to their frequency, which is the number of vibrations that occur per second (in units of Hertz, abbreviated Hz). Humans hear best in the range of 2000–4000 Hz, but our entire audible range is typically 20–20,000 Hz (Gescheider 1997, Figure 4). Vibrations outside of this audible range will not be detected by our ears, but can be detected by many animals. For example, elephants produce sounds below 20 Hz (infrasound) to communicate with other members of their herd (Langbauer et al. 1991, Poole et al. 1988). These low-frequency sounds travel great distances through the air, and through the ground, enabling elephant groups to maintain contact over a range of tens of kilometers. (McComb et al. 2003, Payne et al. 1986, Poole et al. 1988).
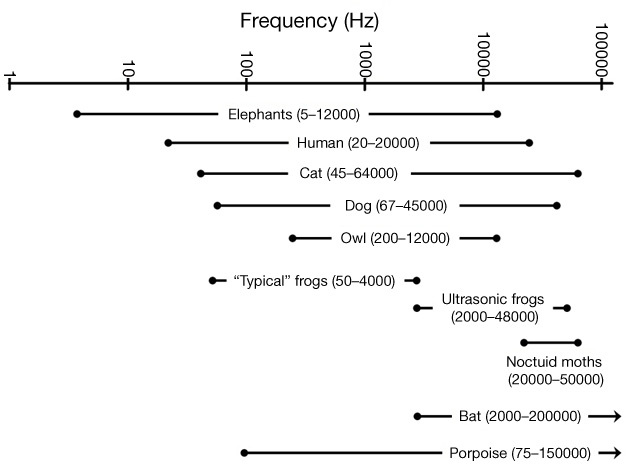
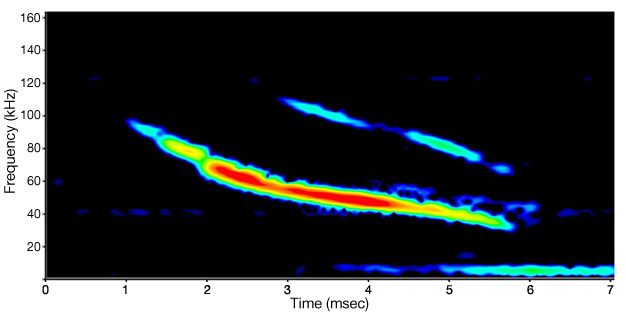
Ultrasound (sound at frequencies of 20,000 Hz or higher) is also produced and/or detected by many species of animals. Cetaceans (Au 2004), rodents (Moles & D'Amato 2000), bats (Fenton 1984), frogs (Feng et al. 2006, Shen et al. 2008), and insects (Fullard 1982, Fullard & Yack 1993, Fullard et al. 1994, Rodríguez & Greenfield 2003), are all known to use ultrasound for a variety of purposes, including communication, predator avoidance, and echolocation. While many studies have been done on this type of sound, one of the best-known examples is the echolocation system of bats. The frequency range of bat echolocation calls varies over 10,000 to 200,000 Hz, depending upon the species (Altringham 1996, Leonard & Fenton 1984). A single bat call may last only a few milliseconds while covering a frequency range of 50,000 Hz or more (Figure 5). High frequency sounds like these do not travel very far, but they do reflect off solid objects, including very small ones (Lawrence & Simmons 1982, Surlykke & Kalko 2008). This allows a bat to detect small targets (e.g., food) from the echoes that reflect from the object. While some echolocating species have been found to use vision for navigation (Bell 1985, Bell & Fenton 1986), even those species generally emit echolocation signals while they are moving, indicating that they still depend on their echolocation abilities (Eklöf & Jones 2003, Masters & Harley 2004). Because their high-frequency calls travel only short distances, a bat's perception of the world is strongly affected by the distances over which its sounds will travel (Lawrence & Simmons 1982, Surlykke & Kalko 2008). This is in marked contrast to how we would perceive the same environment primarily using vision.
Conclusions
The purpose of this article has been to "open your eyes" to the sensory worlds inhabited by other animals, and to realize that these may be very different from the world we perceive. Although we have focused attention primarily on stimuli that humans cannot detect, it is common for the sensory systems of humans and other animals to overlap (Figure 4). In all of the examples presented here, the organisms can hear or see over a broader range then the specific stimulus ranges that were discussed. Most animals (including humans) have receptors that can detect stimuli across a fairly wide range. Thus, to understand the umwelt of an animal, we must learn about the entire range of stimuli it can detect to truly understand their sensory ecology; that is, their sensory systems and how these systems affect the animal's perception of the world (Kevan et al. 2001, McCrea & Levy 1983). In addition, we must remember that in any given situation an animal is likely receiving input from multiple sensory modalities (Dötterl & Vereecken 2010). There are many practical implications of the different sensory systems that have evolved. Studies have shown that the type of sensory stimuli that an organism detects can determine its ability to locate appropriate food (Bell & Fenton 1984, Fenton & Ratcliffe 2004, Siemers & Swift 2006), compete for resources (Caldwell et al. 2010, Mason & Parker 2010), find and attract a mate (Feng et al. 2006, Mason & Parker 2010, Schul & Patterson 2003), or avoid predators (Fullard 1982, Mason & Parker 2010, Schul & Patterson 2003). There are even examples where the evidence suggests that the interaction of sensory systems with a species' environment may actually be driving the evolution of new species (Fenton & Ratcliffe 2004, Kirkpatrick & Price 2008, Seehausen et al. 2008). While each organism inhabits its own umwelt, our ability to study the different types of stimuli that animals use is allowing us to appreciate their perceptual worlds and understand how sensory systems relate to the evolution and ecology of these organisms.
References and Recommended Reading
Akesson, S. & Backman, J. Orientation in pied flycatchers: The relative importance of magnetic and visual information at dusk. Animal Behaviour 57, 819–828 (1999).
Altringham, J. D. Bats: Biology and Behaviour. New York, NY: Oxford University Press, 1996.
Arnold, S. E. J. et al. FReD: The floral reflectance database — a web portal for analyses of flower colour. PLoS ONE 5, e14287 (2010).
Au, W. W. L. "A comparison of the sonar capabilities of bats and dolphins." In Echolocation in Bats and Dolphins, eds. J. A. Thomas, C. F. Moss, & M. Vater (Chicago, IL: University of Chicago Press, 2004): xiii–xxvii.
Bell, G. P. The sensory basis of prey location by the California leaf-nosed bat Macrotus californicus (Chiroptera: Phyllostomidae). Behavioral Ecology and Sociobiology 16, 343–347 (1985).
Bell, G. P. & Fenton, M. B. Visual acuity, sensitivity and binocularity in a gleaning insectivorous bat, Macrotus californicus (Chiroptera: Phyllostomidae). Animal Behaviour 34, 409–414 (1986).
Caldwell, M. S. et al. Vibrational signaling in the agonistic interactions of red-eyed treefrogs. Current Biology 20, 1–6 (2010).
Camperi, M., Tricas, T. C., & Brown, B. R. From morphology to neural information: The electric sense of the skate. PLoS Computational Biology 3, e113 (2007).
Cocroft, R. B. & Rodriguez, R. L. The behavioral ecology of insect vibrational communication. Bioscience 55, 323–334 (2005).
Dangles, O. et al. Variability in sensory ecology: Expanding the bridge between physiology and evolutionary biology. Quarterly Review of Biology 84, 51–74 (2009).
Dötterl, S. & Vereecken, N. J. The chemical ecology and evolution of bee-flower interactions: A review and perspectives. Canadian Journal of Zoology 88, 668–697 (2010).
Dusenbery, D. B. Sensory Ecology: How Organisms Acquire and Respond to Information. New York, NY: W. H. Freeman & Company, 1992.
Ebert, J., Muller, S., & Westhoff, G. Behavioural examination of the infrared sensitivity of ball pythons. Journal of Zoology 272, 340–347 (2007).
Eklöf, J. & Jones, G. Use of vision in prey detection by brown long-eared bats, Plecotus auritus. Animal Behaviour 66, 949–953 (2003).
Feng, A. S. et al. Ultrasonic communication in frogs. Nature 440, 333–336 (2006).
Fenton, M. B. Echolocation: Implications for ecology and evolution of bats. Quarterly Review of Biology 59, 33–53 (1984).
Fenton, M. B. & Ratcliffe, J. Eavesdropping on bats. Nature 429, 612–613 (2004).
Fullard, J. H. Echolocation assemblages and their effects on moth auditory systems. Canadian Journal of Zoology 60, 2572–2576 (1982).
Fullard, J. H., Simmons, J. A., & Saillant, P. A. Jamming bat echolocation: The dogbane tiger moth Cycnia tenera times its clicks to the terminal attack calls of the big brown bat Eptesicus fucus. Journal of Experimental Biology 194, 285–298 (1994).
Fullard, J. H. & Yack, J. E. The evolutionary biology of insect hearing. Trends in Ecology & Evolution 8, 248–252 (1993).
Gescheider, G. A. Psychophysics: The Fundamentals, 3rd ed. Mahwah, NJ: Lawrence Erlbaum Associates, 1997.
Griffin, D. R. Listening in the Dark. New Haven, NJ: Yale University Press, 1958.
Heiligenberg, W. F. Neural Nets in Electric Fish. Cambridge, MA: MIT Press, 1991.
Holland, R. A., Borissov, I., & Siemers, B. M. A nocturnal mammal, the greater mouse-eared bat, calibrates a magnetic compass by the sun. Proceedings of the National Academy of Sciences of the United States of America 107, 6941–6945 (2010).
Kevan, P. G., Chittka, L., & Dyer, A. G. Limits to the salience of ultraviolet: Lessons from colour vision in bees and birds. Journal of Experimental Biology 204, 2571–2580 (2001).
Kirkpatrick, M. & Price, T. In sight of speciation. Nature 455, 601–602 (2008).
Langbauer, W. R., Jr. et al. African elephants respond to distant playbacks of low-frequency conspecific calls. Journal of Experimental Biology 157, 35–46 (1991).
Lawrence, B. D. & Simmons, J. A. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. Journal of the Acoustical Society of America 71, 585–590 (1982).
Leonard, M. L. & Fenton, M. B. Echolocation calls of Euderma maculatum (Vespertilionidae): Use in orientation and communication. Journal of Mammalogy 65, 122–125 (1984).
Mason, R. T. & Parker, R. M. Social behavior and pheromonal communication in reptiles. Journal of Comparative Physiology A 196, 729–749 (2010).
Masters, W. M. & Harley, H. E. "Performance and cognition in echolocating mammals." In Echolocation in Bats and Dolphins, eds. J. A. Thomas, C. F. Moss, & M. Vater (Chicago, IL: University of Chicago Press, 2004): 249–259.
McComb, K. et al. Long-distance communication of acoustic cues to social identity in African elephants. Animal Behaviour 65, 317–329 (2003).
McCrea, K. D. & Levy, M. Photographic visualization of floral colors as perceived by honeybee pollinators. American Journal of Botany 70, 369–375 (1983).
Moles, A. & D'Amato, F. R. Ultrasonic vocalization by female mice in the presence of a conspecific carrying food cues. Animal Behaviour 60, 689–694 (2000).
Nagel, T. What is it like to be a bat? The Philosophical Review 83, 435–450 (1974).
Newman, E. A. & Hartline, P. H. The infrared 'vision' of snakes. Scientific American 246, 116–127 (1982).
Olender T. et al. The canine olfactory subgenome. Genomics 83, 361–372 (2004).
Pappas, T. C., Motamedi, M., & Christensen, B. N. Unique temperature-activated neurons from pit viper thermosensors. American Journal of Physiology - Cell Physiology 287, C1219–C1228 (2004).
Payne, K. B., Langbauer Jr., W. R., & Thomas, E. M. Infrasonic calls of the Asian elephant (Elephans maximus). Behavioral Ecology and Sociobiology 18, 297–301 (1986).
Poole, J. H. et al. The social contexts of some very low frequency calls of African elephants. Behavioral Ecology and Sociobiology 22, 385–392 (1988).
Rodríguez, R. L. & Greenfield, M. D. Genetic variance and phenotypic plasticity in a component of female mate choice in an ultrasonic moth. Evolution 57, 1304–1313 (2003).
Rubene, D. et al. The presence of UV wavelengths improves the temporal resolution of the avian visual system. Journal of Experimental Biology 213, 3357–3363 (2010).
Rüting, T. Jakob von Uexküll — Theoretical biology, biocybernetics and biosemiotics. European Communications in Mathematical and Theoretical Biology 6, 11–16 (2004).
Rutowski, R. L. et al. Diversity in structural ultraviolet coloration among female sulphur butterflies (Coliadinae, Pieridae). Arthropod Structure & Development 36, 280–290 (2007).
Ryer, A. D. The Light Measurement Handbook. Newburyport, MA: International Light, Inc., 1997.
Schul, J. & Patterson, A. C. What determines the tuning of hearing organs and the frequency of calls? A comparative study in the katydid genus Neoconocephalus (Orthoptera, Tettigoniidae). Journal of Experimental Biology 206, 141–152 (2003).
Seehausen, O. et al. Speciation through sensory drive in cichlid fish. Nature 455, 620–626 (2008).
Shen, J-X. et al. Ultrasonic frogs show hyperacute phonotaxis to female courtship calls. Nature 453, 914–917 (2008).
Shettleworth, S. J. Cognition, Evolution, and Behavior. New York, NY: Oxford University Press. 1998.
Sichert, A. B., Friedel, P., & van Hemmen, J. L. Snake's perspective on heat: Reconstruction of input using an imperfect detection system. Physical Review Letters 97, 1–4 (2006).
Siemers, B. M. & Swift, S. M. Differences in sensory ecology contribute to resource partitioning in the bats Myotis bechsteinii and Myotis nattereri (Chiroptera: Vespertilionidae). Behavioral Ecology and Sociobiology 59, 373–380 (2006).
Stebbins, W. C. The Acoustic Sense of Animals. Cambridge, MA: Harvard University Press, 1983.
Strain, G. M. How well do dogs and other animals hear? Baton Rouge, LA: Louisiana State University, 2003–present.
Surlykke, A. & Kalko, E. K. V. Echolocating bats cry out loud to detect their prey. PLoS ONE 3, e2036 (2008).
van Dyke, J. U. & Grace, M. S. The role of thermal contrast in infrared-based defensive targeting by the copperhead, Agkistrodon contortrix. Animal Behaviour 79, 993–999 (2010).
Weindler, P. B. et al. Development of migratory orientation in pied flycatchers in different magnetic inclinations. Animal Behaviour 49, 227–234 (1995).































