« Prev Next »

Sorptives and Sorbents in Soils
Most relevant chemicals can be grouped into three sorptive categories: (a) anionic sorptives that are negatively charged because they have more electrons than protons (e.g., the nutrient orthophosphate, PO43-, is an anion); (b) cationic sorptives that are positively charged because they have fewer electrons than protons (e.g., the divalent cations Ca2+ and Pb2+); and (c) uncharged organic sorptives that exhibit a range of polarities (non-polar to polar) based on the distribution of electrons across the molecule (e.g., the aromatic ring compound, benzene, C6H6, has uniform electron distribution and hence is non-polar, whereas sucrose, C12H22O11, has a non-uniform distribution of electrons and is highly polar). These categories can also be applied to portions of large sorptives that contain a diversity of functional groups. For instance, the antibiotic oxytetracycline contains hydroxyl (R-OH) groups that can lose a proton (deprontonate) to become negatively charged (R-O-), amino (R-NH2) groups that can protonate to form positively-charged functional groups (R-NH3+), and un-charged, non-polar aromatic (-Ar-) ring structures. Thus, depending on the solution pH, oxytetracycline can exhibit behavior consistent with any of these three sorptive categories.In a similar manner, we can broadly conceptualize the reactive surface sites on sorbents as positively charged, negatively charged, or un-charged (polar or non-polar) in nature. The major solid phase materials (sorbents) in soils are layer silicate clays, metal-(oxyhydr)oxides, and soil organic matter (SOM). Layer silicate clays are primarily negatively charged because their stacks of aluminum-oxygen and silicon-oxygen sheets are often chemically substituted by ions of lower valance. In many soils, they represent the largest source of negative charge. Metal-(oxyhydr)oxides are variably charged because their surfaces become hydroxylated when exposed to water (Liu et al. 1998) and assume anionic, neutral, or cationic forms based on the degree of protonation (≡M-O-, ≡MOH or ≡MOH2+, where ≡M represents a metal bound at the edge of a crystal structure), which varies as a function of solution pH. Thus, these variably charged minerals adopt a net positive surface charge at low pH and a net negative surface charge at high pH (see Qafoku et al. 2004 for a recent review of variably charged soil minerals). SOM includes living and partially decayed (non-living) materials as well as assemblages of biomolecules and transformation products of organic residue decay known as humic substances. SOM contains a multitude of reactive sites ranging from potentially anionic hydroxyls (R-OH) and carboxylic groups (R-COOH) to cationic sulfhydryl (R-SH) and amino groups (R-NH2), as well as aromatic (-Ar-) and aliphatic ([-CH2-]n) moieties that are the principal un-charged, non-polar regions of the soil solid phase. Variations in the abundance, surface area and chemical composition of these three sorbents significantly influence the sorption characteristics of a given soil (Johnston & Tombácz 2002).
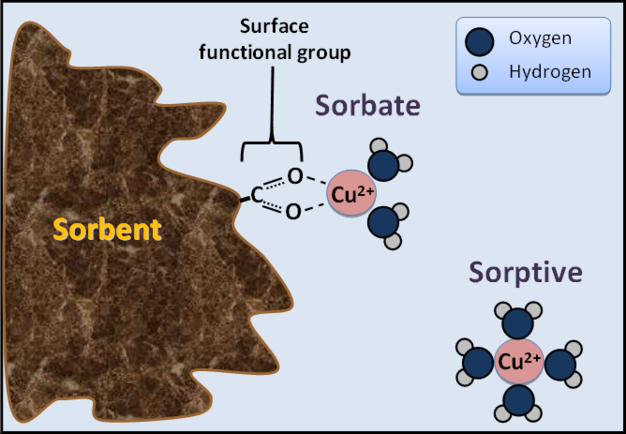
Principal Factors Governing Sorption in Soils
Sorptive Concentration
A common method for assessing sorption characteristics of a soil is to measure the relationship (or distribution) between the equilibrium concentration of the sorptive (Ceq in mol L-1) and the sorbate (Γads in mol kg-1) across a range of sorptive concentrations while holding temperature and other parameters constant. The resulting dataset is termed a sorption isotherm (Figure 2) and is often used in a predictive fashion to describe sorption behavior. For many organic compounds and ions in solution at low concentration (Chiou 2002), this relationship is linear and summarized by a distribution coefficient (Kd; L kg-1):
[1]
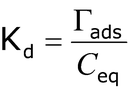
However, at higher ion concentrations the relationship between Γads and Ceq can exhibit significant nonlinearity (Huang et al. 2002), often approaching a maximum sorption plateau after which subsequent increases in sorptive concentration do not result in additional sorption. Many sandy agricultural soils are near their maximum sorption capacity for phosphorus (P) as a result of long-term fertilization practices and/or a naturally low affinity for P.
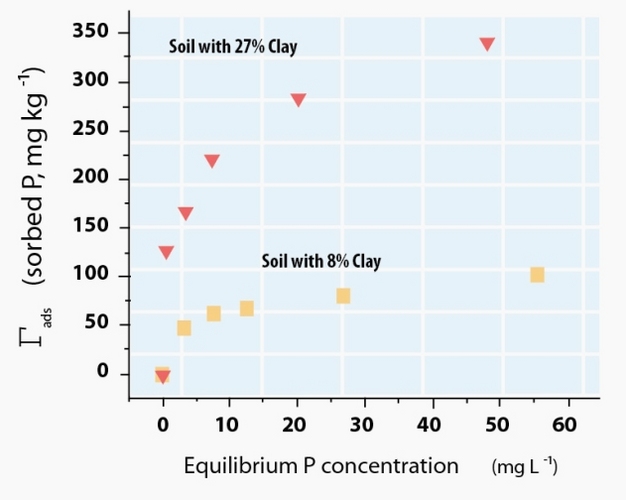
Sorptive and Sorbent Charge
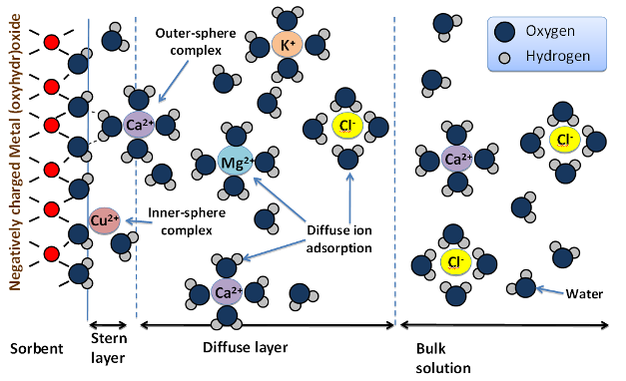
Solution pH
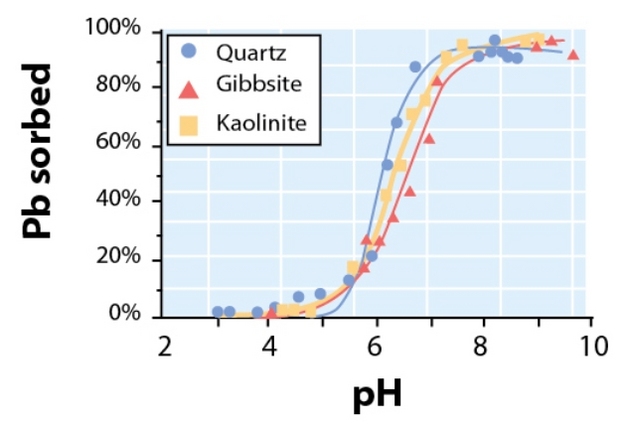
Solution pH can have a pronounced effect on sorption by influencing both the magnitude and sign of sorptive and sorbent charge. As the solution pH increases, sorbent hydroxyl and carboxyl functional groups of metal-(oxyhydr)oxides and SOM deprontonate. In turn, this increases negative charge density on the sorbent thus facilitating cation adsorption (Figure 4), while decreasing anion adsorption. Consequently, the ability of a soil to retain cations (e.g., Ca2+, Mg2+, K+, etc. — the sum of which represents the cation exchange capacity) generally improves when soil pH is increased through liming. Sorptives that can undergo acid dissociation or hydrolysis reactions also exhibit strong pH-dependent sorption trends (Essington 2004). For instance, the previously mentioned antibiotic oxytetracycline (OTC) is predominately positively charged at pH values below its first acid-dissociation constant (pKa1 = 3.3), net neutral between pH 3.3 to 7.3 (pKa2 = 7.3), and negatively charged above 7.3. Consequently, OTC sorption is greater at lower pH than at higher pH in soils containing layer silicate and organic matter sorbents (Sassman & Lee 2005), which can remain negatively charged below pH 3. In contrast, OTC sorption to metal-(oxyhydr)oxides-which are generally positively charged below pH 8 — is often greatest between pH 7.3 and 8 when OTC is negatively charged and the metal-(oxyhydr)oxide is still positively charged (Figueroa & MacKay 2005).
Sorptive Size
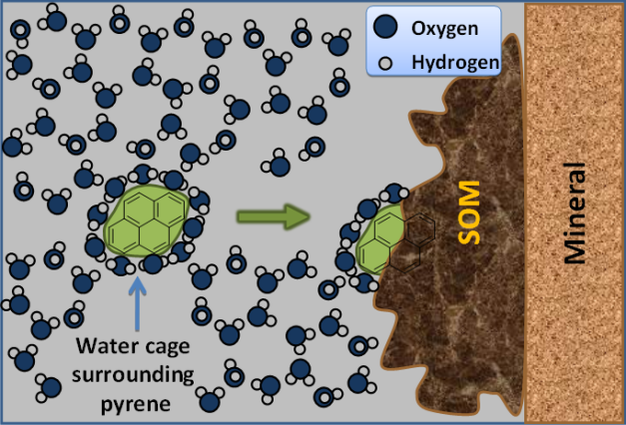
Sorption of Organic Molecules: Additional Considerations
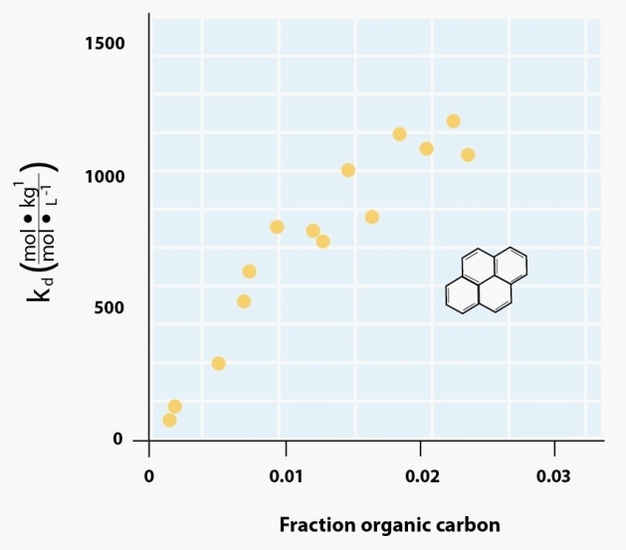
Sorption of un-charged compounds with polar functional groups — particularly those containing oxygen or nitrogen atoms — are strongly influenced by hydrogen bonding between the sorbate and sorbent. Hydrogen bonding develops due to interaction of a hydrogen atom in a polar bond and an unshared electron pair in an electronegative atom in close proximity. For example, a sorbate ketone group (C=O) can form a hydrogen bond with a hydroxylated surface functional group on a mineral surface. Both van der Waals forces and hydrogen bonding are additive across the molecule. Thus, as size of the sorbate increases, these attractive forces become progressively more important for sorbate retention to the soil (Schwarzenbach 2003). For example, the soil distribution coefficient (Kd) for the single aromatic ring compound benzene is smaller than the Kd value for pyrene, which has four fused aromatic rings (Chiou et al. 1998). The preferential sorption of humic substances with high molecular weight over those with lower molecular weight can be explained in similar terms (Zhou et al. 2001).
Conclusion
Aaron Thompson1* and Keith W. Goyne2
[1]Department of Crop and Soil Sciences, University of Georgia, Athens, Georgia 30602, USA.
[2]Department of Soil, Environmental and Atmospheric Sciences, University of Missouri, Columbia, MO 65211, USA
* Corresponding author: AaronT@uga.edu; 706-410-1293.
Glossary
adsorption: The process through which ions or molecules are removed from solution and accumulate on solid constituents at the solid-liquid interface.
Cation Exchange Capacity (CEC): Sum of cations that can be displaced (exchanged) into solution from the soil solid phase when the soil is equilibrated with a dilute salt solution; typically expressed in units of cmolc kg-1.
desorption: The release of ions or molecules from solids into solution.
diffuse layer: The region of ion adsorption near a sorbent surface that is subject to diffusion with the bulk solution. Diffuse layer ions are not immediately adjacent the surface, but rather are distributed between the inner, Stern layer ions and the bulk solution by balance of electrostatic attraction to the sorbent and diffusion away form the sorbent.
functional group: Atom or group of atoms in a chemical structure that impart a particular chemical characteristic to the compound. A specific functional group (e.g., R-COOH) typically behaves in a similar manner on all chemical structures, although its behavior is modified to a degree by the bonding environment.
humic substances: Dark, complex, heterogeneous mixtures of organic materials that form in geomedia from microbial transformations and chemical reactions that occur during the decay of organic biomolecules, polymers, and resides.
hydration sphere: Shell of water molecules surrounding an ion in solution.
hydrogen bond: Intermolecular attraction between a hydrogen atom in a polar bond with an unshared electron pair of an electronegative atom in close proximity.
hydrophobic effect: The attraction of nonionic, non-polar compounds to surfaces that occurs due to the thermodynamic drive of these molecules to minimize interactions with water molecules.
inner-sphere adsorption complex: Sorption of an ion or molecule to a solid surface where waters of hydration are distorted during the sorption process and no water molecules remain interposed between the sorbate and sorbent.
layer silicate clay: Clay minerals composed of planes of aluminum (AlIII) or magnesium (MgII) in octahedral coordination with oxygen and planes of silica (SiIV) in tetrahedral coordination to oxygen. Substitution of AlIII for SiIV in the tetrahedral plane or substitution of MgII or FeII for AlIII in the octahedral plane (isomorphic substitution) results in a permanent charge imbalance (i.e., structural charge) that must be satisfied through cation adsorption.
metal (oxyhydr)oxide: Minerals composed of various structural arrangements of metal cations-principally AlIII, FeIII and MnIV-in octahedral coordination with oxygen or hydroxide anions. These minerals are dissolution byproducts of mineral weathering and they are often found as coatings on layer silicates and other soil particles.
outer-sphere adsorption complex: Sorption of an ion or molecule to a solid surface where waters of hydration are interposed between the sorbate and sorbent.
precipitation: Formation of an insoluble product that occurs via reactions between ions or molecules in solution.
soil organic matter: Living and partially decayed (non-living) materials as well as assemblages of biomolecules and transformation products of organic residue decay known as humic substances.
sorbate: Ions or molecules that have accumulated on or within a solid due to a sorption reaction.
sorbent: The solid phase constituent participating in a sorption reaction. The solid phase may be more specifically referred to as an absorbent or adsorbent if the mechanism of removal is known to be absorption or adsorption, respectively.
sorption: Removal of a compound from solution by solid phase constituents. This term is often used when the mechanism of removal (adsorption, absorption, or precipitation) is unknown.
sorption isotherm: Graphical representation of surface excess (i.e., the amount of substance sorbed to a solid) relative to sorptive concentration in solution after reaction at fixed temperature, pressure, ionic strength, pH, and solid-to-solution ratio.
sorptive: Ions or molecules in solution that could potentially participate in a sorption reaction.
Stern layer: The layer of ions adsorbed immediately adjacent to a charged sorbent surface. Ions in the Stern layer can be directly bonded to the sorbent through covalent and ionic bonds (inner-sphere complexes) or held adjacent to a sorbent through strictly electrostatic forces in outer-sphere complexes.
van der Waals forces: Intermolecular attractive forces that arise between nonionic, non-polar molecules due to dipole-dipole interactions and instantaneous dipole interactions (London dispersion forces).
References and Recommended Reading
Borda, M. J. & Sparks, D. L. "Kinetics and mechanisms of sorption-desorption in soils: A multiscale assessment," in Biophysical-Chemical Processes of Heavy Metals and Metalloids in Soil Environments, 97-124, eds. A. Violette, P. M. Huang, & G. M. Gadd, New York: Wiley, 2008.
Brown Jr, G. E. & Calas, G. Environmental mineralogy - Understanding element behavior in ecosystems. Comptes Rendus Geoscience 343, 90-112 (2011).
Brown, G. E. & Sturchio, N. C. "An overview of synchrotron radiation applications to low temperature geochemistry and environmental science," in Applications of Synchrotron Radiation in Low-Temperature Geochemistry and Environmental Sciences, Volume 49, 1-115, Reviews in Mineralogy & Geochemistry, eds. P. A. Fenter et al., Chantilly, VA: Mineralogical Society of America, 2002.
Chiou, C. T. et al. Partition characteristics of polycyclic aromatic hydrocarbons on soils and sediments. Environmental Science & Technology 32, 264-269 (1998).
Chiou, C. T. Partition and Adsorption of Organic Contaminants in Environmental Systems. New York, NY: Wiley, 2002.
Chorover, J. & Brusseau, M. L. "Kinetics of sorption-desorption," in Kinetics of Water-Rock Interaction, eds. S. L. Brantley, J. D. Kubicki, & A. F. White, 109-149, New York: Springer, 2009..
Essington, M. E. Soil and Water Chemistry. Boca Raton, FL: CRC Press, 2004.
Figueroa, R. A. & MacKay, A. A. Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environmental Science & Technology 39, 6664-6671 (2005).
Goyne, K. W. et al. Phosphorus and nitrogen sorption to soils in the presence of poultry litter-extracted dissolved organic matter. Journal of Environmental Quality 37, 154-163 (2008).
Huang, W. et al. Effects of organic matter heterogeneity on sorption and desorption of organic contaminants by soils and sediments. Applied Geochemistry 18, 955-972 (2003).
Hesterberg, D. et al. X-ray microspectroscopy and chemical reactions in soil microsites. Journal of Environmental Quality 40, 667-678 (2011).
Johnston, C. T. & Tombácz, E. "Surface chemistry of soil minerals," in Soil Mineralogy With Environmental Applications, eds. J. B. Dixon & D. G. Schulze, 37-67, Madison, WI: Soil Science Society of America, 2002.
Kaplan, D. I. Influence of surface charge of an Fe-oxide and an organic matter dominated soil on iodide and pertechnetate sorption. Radiochimica Acta 91, 173-178 (2003).
Lehmann, J. et al. Spatial complexity of soil organic matter forms at nanometre scales. Nature Geoscience 1, 238-242 (2008).
Liu, P. et al. Reaction of water vapor with α-Al2O3(0001) and α -Fe2O3(0001) surfaces: Synchrotron X-ray photoemission studies and thermodynamic calculations. Surface Science 417, 53-65 (1998).
Maurice, P. A. Environmental Surfaces and Interfaces From The Nanoscale To The Global Scale. New York, NY: Wiley, 2009.
Means, J. C. et al. Sorption of polynuclear aromatic hydrocarbons by sediments and soils. Environmental Science & Technology 14, 1524-1528 (1980).
Pignatello, J. J. & Xing, B. Mechanisms of slow sorption of organic chemicals to natural particles. Environmental Science and Technology 30, 1-11 (1995).
Qafoku, N. P. et al. "Variable charge soils: Their mineralogy, chemistry and management," in Advances in Agronomy, Volume 84, ed. D. L. Sparks, 159-215, New York: Academic Press, 2004.
Sassman S. A. & Lee, L. S. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange. Environmental Science & Technology 39, 7452-7459 (2005).
Schwarzenbach, R. P., Gschwend, P. M. & Imboden, D. M. Environmental Organic Chemistry, 2nd ed. New York, NY: Wiley, 2003.
Singh, B. & Gräfe, M. Synchrotron-Based Techniques in Soils and Sediments. New York, NY: Elsevier, 2010.
Sollins, P. et al. Nutrient mobility in variable and permanent-charge soils. Biogeochemistry 6, 181-199 (1988).
Sparks, D. L. Environmental Soil Chemistry, 2nd ed. San Deigo, CA: Academic Press, 2003.
Sposito, G. The Surface Chemistry of Natural Particles. New York, NY: Oxford University Press, 2004.
Sposito, G. The Chemistry of Soils, 2nd ed. New York, NY: Oxford University Press, 2008.
Sutton, R. & Sposito, G. Molecular structure in soil humic substances: The new view. Environmental Science & Technology 39, 9009-9015 (2005).
Thompson, H. S. On the absorbent power of soils. Journal of the Royal Agricultural Society England 11, 68-74 (1850).
Way, J. T. On the power of soils to absorb manure. Journal of the Royal Agricultural Society England 11, 313-379 (1850).
Young, T. M. & Weber Jr, W. J. "Sorption and desorption rates for neutral organic compounds in soils," in Chemical Processes in Soils, 519-562, eds. M. A. Tabatabai & D. L. Sparks, Madison, WI: Soil Science Society of America, 2005.
Zachara, J. M. et al. Geochemical processes controlling migration of tank wastes in Hanford's vadose zone. Vadose Zone Journal 6, 985-1003 (2007).
Zhang, Z. et al. Ion adsorption at the rutile-water interface: Linking molecular and macroscopic properties. Langmuir 20, 4954-4969 (2004).
Zhou, Q. et al. Size fractionation upon adsorption of fulvic acid on goethite: Equilibrium and kinetic studies. Geochimica et Cosmochimica Acta 65, 803-812 (2001).






























