« Prev Next »

Animals occupy diverse types of biomes and aquatic environments. A key factor in their success is the ability for many species to adapt to external environmental fluctuations and maintain a consistent internal environment. Cannon (1932) described the capacity for an animal to regulate its internal environment as the product of a suite of physiological processes, called homeostasis. Homeostatic mechanisms are dynamic and regulate many different parameters in the animal body (e.g., pH, dissolved oxygen, glucose concentration). The focus of this article concerns homeostatic control of body temperature (TB) in animals. Internal temperature changes may adversely affect many aspects of animal physiology, including enzyme function, muscle activity, and energy metabolism.
Types of Thermoregulation
There are two primary responses to fluctuating ambient temperatures (TA) exhibited by animals: poikilothermy and homeothermy (Figure 1). Because poikilotherms lack the physiological means to generate heat, the body temperature of these animals tends to conform to that of the outside environment in the absence of any behavioral intervention. Examples of poikilotherms include the "cold-blooded" animals (Kearney et al. 2009), such as most fish, amphibians, and reptiles. On the other hand, homeotherms have specific physiological adaptations for regulating their body temperatures; body temperatures of homeotherms do not fluctuate as much as those of poikilotherms. Indeed, all homeotherms maintain high body temperatures in the range of 36 to 42oC (Ivanov 2006) and include the "warm-blooded" animals, such as birds and mammals.
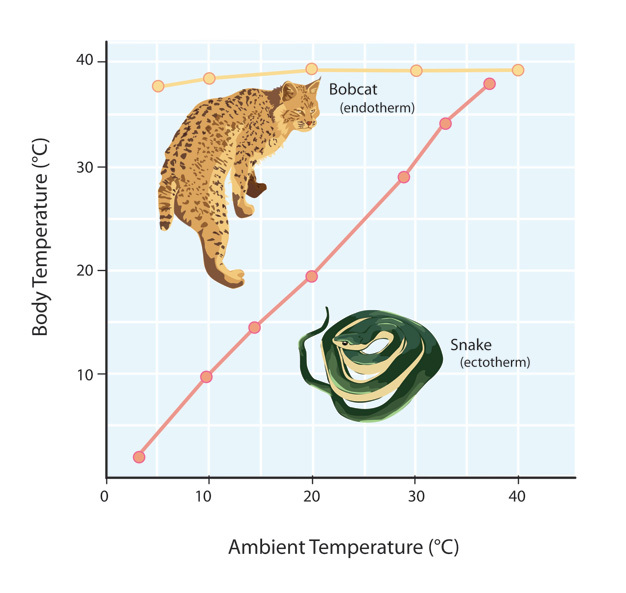
Poikilotherms are also known as ectotherms because their body heat is derived exclusively from their external environments. This external thermal dependence enables them to employ behavioral thermoregulation by 1) shuttling between areas with lower and higher temperatures and 2) changing body positions to adjust heat exchange via conduction and radiation (Kiefer et al. 2007). For example, wood turtles (Glyptemys insculpta) move daily into forest clearings to bask and elevate their body temperatures but return to streams at night because the water temperature does not drop as much as does the air temperature (Dubois et al. 2009). Behavioral thermoregulation in poikilotherms can result in sustained average body temperatures that are optimal for crucial metabolic activities, such as feeding and locomotion (Huey & Kingsolver 1989). Reiserer et al. (2008) describe groups of neonate sidewinder rattlesnakes (Crotalus cerastes) that aggregate as "ball" groups during the time period leading up to the first shedding. The ball core temperature remained stable (mean of 31.94 ± 2oC) over a 5-hour period during the hottest part of the day, while temperatures measured outside the balls exceeded 42oC, the lethal critical maximum for these snakes.
Melanistic polymorphism among related species of lizards may be related to thermoregulation. In cordylid (Cordylus) lizards, melanistic species warm up more quickly than do lighter-colored species with higher thermal reflectance (Clusella-Trullas et al. 2009). This heat retention helps these lizards remain active under cold conditions. Other kinds of lizards, such as Amphibolurus decresii and Anolis carolinensis, exhibit labile body coloration but the color choice is not related to thermal condition but, instead, functions in social or camouflage contexts (Gibbons & Lillywhite 1981, Greenberg & Crews 1990). For example, the agamid lizard Pseudotrapelus sinaitus uses body color changes to signal to conspecifics and not to thermoregulate (Norfolk et al. 2010).
Homeotherms also use behavioral thermoregulation (i.e., habitat choice) to adjust their body temperatures (Jackson et al. 2009) but, unlike poikilotherms, they do not depend solely on the outside environment as a source of body heat. Instead, homeotherms use physiological mechanisms to regulate their body temperatures independently from ambient temperatures. When low ambient temperatures threaten to overcool the regulated body temperature, homeotherms have several strategies to supplement and conserve body heat. Isometric contraction of skeletal muscles, called shivering, transfers mechanical heat to the body core while vasoconstriction of peripheral vessels reduces heat loss from the integument (Ivanov 2006). Heinrich (1979) described how shivering by bumblebees in cold ambient temperatures increased thoracic body temperatures to the level required for flight muscle activity (i.e., 30–40oC). In addition, some homeotherms are adapted for non-shivering thermogenesis, a metabolic process in which brown adipose tissue is catabolized not for ATP synthesis, but for heat production (Grigg et al. 2004). As ambient temperatures increase, homeotherms use evaporative cooling through sweating and/or panting to regulate body temperatures, and also vasodilate surface blood vessels to promote heat loss (Robertshaw 2006).
Thermoneutral Zone
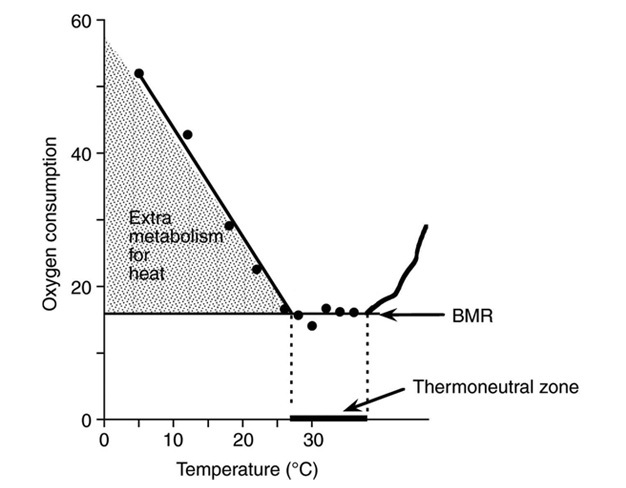
A homeotherm's basic needs are met through basal metabolism, a form of metabolism that does not involve physiological thermoregulation because external temperatures do not exceed comfortable limits (Ivanov 2006). The range of temperatures associated with basal metabolism comprises the thermoneutral zone (Figure 2). When ambient temperatures either exceed or fall below the thermoneutral zone, physiological strategies like those described above are deployed to prevent the body temperature from changing. However, whenever an animal is thermoregulating to prevent overheating or overcooling, the non-basal metabolic rate increases. This increase in metabolic rate constrains the limits of what temperatures can be tolerated beyond the thermoneutral zone, especially the upper temperature limit. In general, homeotherms utilize behavioral means to keep themselves in the thermoneutral zone.
Heterothermy
Even though homeotherms regulate their body temperatures around a specific set point (Cabanac 2006), the body temperature of most homeotherms is not completely uniform. Heterothermy describes variations in body temperature along both spatial and temporal scales. For example, animal body temperature is usually warmest at the core but may be much lower in the extremities. The extremities are usually allowed to cool in homeotherms, while the body core temperature can be conserved by warming the blood returning from the extremities through counter-current exchange. In jackrabbits (Figure 3), the ears are allowed to warm above core body temperature, in order to facilitate body heat dissipation by radiation (Hill et al. 1980).

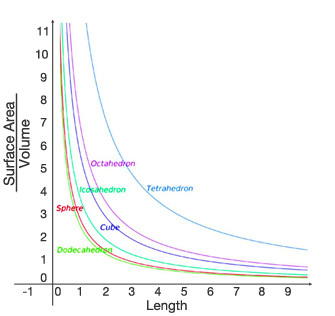
Temporal heterothermy refers to body temperature differences in the same animal over time. Many animals change their insulation values seasonally (Soppela et al. 1986) in order to either conserve or release body heat. Other homeotherms may temporarily use fever in response to pathogen presence (Kluger 1991). A special case of temporal heterothermy involves animals that not only adjust their body temperature but also adjust their metabolic rates. These adjustments are necessary because energy resources may not be available to fuel the same basal metabolic rates at all times. For example, black-tailed prairie dogs (Cynomys ludovicianus) remain active during the winter but periodically undergo shallow bouts of reduced metabolic rate and hypothermia, a state called torpor, when conditions are too harsh to permit exposure (Kiefer et al. 2007). In small bodied-homeotherms, heat loss across their higher surface area relative to body volume (Figure 4) is so great that they must undergo daily metabolic adjustments. Daily torpor is a frequent reduction in metabolic rate that conserves limited energy reserves that would otherwise have to be diverted to thermoregulation when the animal could not forage to keep up energy stores (Edelman & Koprowski 2007, Hwang et al. 2006).
Control of Thermoregulation
Life in Extreme Temperatures
Tolerance to extreme temperatures is not just a feat for homeotherms. Some poikilotherms are capable of doing this as well. Several species of amphibians and fish are able to withstand freezing temperatures. For example, the wood frog (Rana sylvatica) can freeze solid and remain in a state of suspended animation until the spring thaw. Wood frogs and other freezing-tolerant animals utilize cryoprotectants, such as glucose and urea, to dehydrate cells and limit freezing to extracellular spaces (Constanzo & Lee 2008).
Conclusion
Animals exhibit many different types of thermoregulatory strategies, but is one better than another? The answer depends upon what we are measuring. Homeotherms have a higher metabolic rate and are more active over a wider range of temperatures than are poikilotherms (Clarke & Pörtner 2010), but this high metabolic activity comes at a cost, as homeotherms need to divert a large proportion of their energy intake to thermoregulation. This constrains where they can live unless they are able to secure and/or store a lot of energy sources. Torpor is an adaptation that can preclude this problem. Poikilotherms, with their lower metabolic rates, can feed less and are therefore more likely to be able to live in resource-poor environments (Grigg et al. 2004)
Are there limits to the thermoregulatory abilities of animals in the face of climate change (Kearney et al. 2009)? Both homeotherms and poikilotherms have remarkable adaptations for living in environments that pose temperature challenges. But these adaptations for variable but predictable thermal conditions may not be able to compensate for climate change. For example, late summer steelhead salmon (Oncorhynchus mykiss) migration is now only possible by fish that are able to access cooler thermal refugia during their runs. Climate change may uniformly increase water temperature and reduce recruitment (Keefer et al. 2009). Species that are distributed along areas with a wide range of temperature extremes may have populations that show plasticity in thermoregulatory behavior. For example, Lehmer et al. (2006) describe an unusual population of black-tailed prairie dog (Cynomys ludovicianus) that hibernates in contrast to other populations of the same species that undergo short bouts of torpor during the same time. The reduced activity in the hibernating population may be the result of unusually dry and cold conditions experienced in this portion of the range of the species. Plasticity in thermoregulatory behavior is also evident from populations of grasshoppers (Samietz et al. 2005) and restinga lizards (Kiefer et al. 2007) that increase active thermoregulation, such as behavioral thermoregulation, according to local conditions. However, an "ecological trap" occurs when a behavior that is adaptive in one context, such as thermoregulation, has a negative consequence in a different context, such as reproduction. Steelhead salmon that tary too long in thermal refugia may exceed their energy reserves necessary for a long migration (Keefer et al. 2009). In sockeye salmon (Oncorhynchus nerka), the high temperature extremes of their altered environments force them to select habitats where they become more vulnerable to predation and less likely to reproduce.
References and Recommended Reading
Cannon, B. & Nedergard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology 214, 242–253 (2011).
Cannon, W. B. The Wisdom of the Body. New York, NY: W. W. Norton and Company, 1932.
Clarke, A. & Pörtner, H-O. Temperature, metabolic power and the evolution of endothermy. Biological Reviews 85, 707–727 (2010).
Clusella-Trullas, S. et al. Thermal benefits of melanism in cordylid lizards: A theoretical and field test. Ecology 90, 2297–2312 (2009).
Costanzo, J. P. & Lee, Jr, R. E. Urea loading enhances freezing survival and postfreeze recovery in a terrestrially hibernating frog. The Journal of Experimental Biology 211, 2969–2975 (2008).
Díaz, M. & Becker, D. E. Thermoregulation: Physiological and clinical considerations during sedation and general anesthesia. Anesthesia Progress 57, 25–33 (2010).
Donaldson, M. R. et al. Limited behavioural thermoregulation by adult upriver-migrating sockeye salmon (Oncorhynchus nerka) in the Lower Fraser River, British Columbia. Canadian Journal of Zoology 87, 480–490 (2009).
Dubois, Y. et al. Thermoregulation and habitat selection in wood turtles Glyptemys insculpta: Chasing the sun slowly. Journal of Animal Ecology 78, 1023–1032 (2009).
Edelman, A. J. & Koprowski, J. L. Communal nesting in asocial Abert’s squirrels: The role of social thermoregulation and breeding strategy. Ethology 113, 147–154 (2007).
Ellis, D. J. et al. Circadian rhythm of behavioral thermoregulation in the sleepy lizard (Tiliqua rugosa). Herpetologica 62, 259–265 (2006).
Gibbons, J. R. H. & Lillywhite, H. B. Ecological segregation, color matching, and speciation in lizards of the Amphibolurus decresii species complex (Lacertilia: Agamidae). Ecology 62, 1573–1584 (1981).
Greenberg, N. & Crews, D. Endocrine and behavioral responses to aggression and social dominance in the green anole lizard, Anolis carolinensis. Hormonal Behavior 18, 1–11 (1990).
Grigg, G. C. et al. The evolution of endothermy and its diversity in mammals and birds. Physiological and Biochemical Zoology 77, 982–997 (2004).
Heinrich, B. Bumblebee Economics. Cambridge, MA: Harvard University Press, 1979.
Hill, R. W. et al. Pinna temperature in exercising jackrabbits, Lepus californicus. Journal of Mammalogy 61, 30–38 (1980).
Huey, R. B. & Kingsolver, J. G. Evolution of thermal sensitivity of ectotherm performance. Trends in Ecology and Evolution 4, 131–135 (1989).
Hwang, Y. T. et al. Energetic consequences and ecological significance of heterothermy and social thermoregulation in striped skunks (Mephitis mephitis). Physiological and Biochemical Zoology 80, 138–145 (2007).
Ivanov, K. P. The development of the concepts of homeothermy and thermoregulation. Journal of Thermal Biology 31, 24–29 (2006).
Jackson, C. R. et al. Insights into torpor and behavioural thermoregulation of the endangered Juliana’s golden mole. Journal of Zoology 278, 299–307 (2009).
Kearney, M. et al. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proceedings of the National Academy of Sciences of the United States of America 106, 3835–3840 (2009).
Keefer, M. L. et al. Behavioral thermoregulation and associated mortality trade-offs in migrating adult steelhead (Onyrhynchus mykiss): Variability among sympatric populations. Canadian Journal of Fisheries and Aquatic Sciences 66, 1734–1747 (2009).
Kiefer, M. C. et al. Thermoregulatory behavior in Tropidurus torquatus (Squamata, Tropiduridae) from Brazilian coastal populations: An estimate of passive and active thermoregulation in lizards. Acta Zoologica 88, 81–87 (2007).
Kluger, M. J. Fever: Role of pyrogens and cryogens. Physiological Review 71, 93–127 (1991).
Lehmer, E. M. et al. Extreme plasticity in thermoregulatory behaviors of free-ranging black-tailed prairie dogs. Physiological and Biochemical Zoology 79, 454–467 (2006).
Lutterschmidt, D. I. et al. Melatonin and thermoregulation in ectothermic vertebrates: A review. Canadian Journal of Zoology 81, 1–13 (2003).
Norfolk, O. et al. A comparative study of two agamid lizards, Laudakia stellio and Pseudotrapelus sinaitus. Egyptian Journal of Biology 12, 27–43 (2010).
Ostrowski, S. et al. Heterothermy and the water economy of free-living Arabian oryx (Oryx leucoryx). Journal of Experimental Biology 206, 1471–1478 (2003).
Reiserer, R. S. et al. Dynamic aggregations of newborn sibling rattlesnakes exhibit stable thermoregulatory properties. Journal of Zoology 274, 277–283 (2008).
Robertshaw, D. Mechanisms for the control of respiratory evaporative heat loss in panting animals. Journal of Applied Physiology 101, 664–668 (2006).
Samietz, J. et al. Altitudinal variation in behavioural thermoregulation: Local adaptation vs. plasticity in California grasshoppers. Journal of Evolutionary Biology 18, 1087–1096 (2005).
Silva, J. E. Thermogenic mechanisms and their hormonal regulation. Physiological Reviews 86, 435–464 (2006).
Soppela, P. et al. Thermoregulation in reindeer. Rangifer Special Issue 1, 273–278 (1986).






























