Kanjaksha Ghosh has seen more than a thousand people with haemophilia since he became a physician. But he has always wondered why some patients bleed spontaneously and develop crippling joint damage whereas others barely seem to be affected.
Ghosh, who heads the National Institute of Immunohaematology in Mumbai, India, remembers a soldier who had been fighting insurgents in the northeast of the country. The man's brother was almost bedridden by haemophilia, but the soldier's symptoms were so mild that he did not even realize that he had the disease until he was shot on the battlefield.
In the 1990s, Ghosh began trying to work out why such discrepancies existed by studying families like the soldier's. When he delved into the genomes of those with a milder disease, he often saw not just a mutation in the affected clotting-factor gene, but also a mutation in another gene—the first causing haemophilia, the tendency to bleed, and the second causing thrombophilia, the tendency to clot. Ghosh's research leads to the conclusion that a patient with haemophilia who co-inherits a thrombophilic gene bleeds less than one without that mutation.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
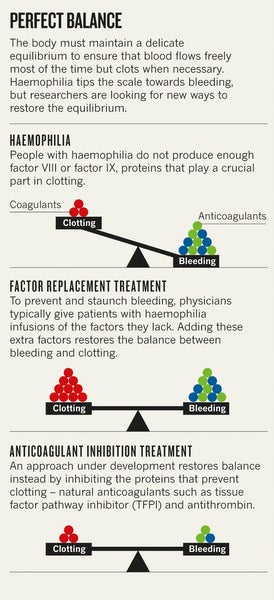
Blood coagulation is regulated by one set of proteins that causes clotting and another set that prevents it. Too little clotting ability leads to bleeding disorders. Too much leads to vessel-blocking clots that can cause strokes and heart attacks. Existing haemophilia treatments tip the balance towards clotting by adding what the body lacks—the clotting factor that is missing or defective. But natural human experiments such as Ghosh's soldier suggest an alternative strategy to treat the disease. Rather than boosting the factors that promote clotting, researchers might instead disable the anticoagulation machinery that prevents clotting.
In the past few years, three drug companies have moved compounds aimed at inhibiting anticoagulation into clinical trials. The hope is that these therapies will be as effective as existing treatments and much more convenient. Rather than receiving multiple infusions of protein replacement each week, patients might be able to control their bleeding with long-lasting injections.
Target practice
The complex cascade that results in the formation of a clot begins when a blood vessel is injured. Several proteins hold the process in check to prevent clots from forming where they are not needed. One such protein, tissue factor pathway inhibitor (TFPI), impedes the initiation of coagulation. Studies published over the past two decades suggest that blocking this protein can promote clotting, which could curb bleeding in people with haemophilia.
The Danish pharmaceutical company Novo Nordisk in Bagsvaerd began working on an antibody designed to inhibit TFPI in the 1990s. Its researchers showed that this antibody could speed up clot formation in blood plasma from people with haemophilia. They also found that it could shorten bleeding time and hasten clotting in rabbits with induced haemophilia. These results seemed promising, but Novo Nordisk began pursuing other strategies to treat haemophilia, and research to develop an anti-TFPI antibody was halted.
In 2006, Novo Nordisk decided to look for therapies that could be injected under the skin and revived the programme. By 2010, the company had launched a clinical trial in Europe and Asia to test the safety of an anti-TFPI monoclonal antibody called concizumab. The researchers administered the antibody either intravenously or subcutaneously to 28 healthy volunteers and 24 people with haemophilia. Preliminary results presented in 2013 at the International Society on Thrombosis and Haemostasis meeting in Amsterdam suggest that concizumab is safe, and that it can improve coagulation. Participants did not report any severe adverse events, although one of the healthy volunteers in the group receiving the highest dose of concizumab developed a small blood clot that disappeared on its own.
The company hopes to launch a second study in mid-2015 to determine the appropriate dose before moving on to test the efficacy of the treatment. “We have liked TFPI as a target for a long time,” says Ida Hilden, scientific director of Novo Nordisk's concizumab project.
Drug company Baxter International, based in Deerfield, Illinois, sells recombinant clotting factors for treating haemophilia and also has its sights on TFPI. In the same year that Novo Nordisk launched its concizumab trial, Baxter struck a deal to purchase a suite of haemophilia-related assets from the former therapeutics company Archemix. Those assets included a therapy designed to inhibit TFPI that had already entered a safety study in the United Kingdom. This therapy was an aptamer, a small strand of nucleotides designed to inhibit TFPI's activity by binding to it, much like an antibody.
The compound, known as BAX 499, performed well in animal studies but failed to deliver in humans. In 2012, Baxter halted the trial due to an increased number of bleeding events. The failure came as a shock. “We did extensive safety studies in monkeys,” says Fritz Scheiflinger, vice-president of research and innovation at Baxter BioScience in Vienna. “We gave huge amounts of aptamer over six months”, yet there were no signs that the compound was unsafe, he says.
Scheiflinger and his colleagues think that they now have an explanation for this strange effect. TFPI lasts no more than a couple of hours in the bloodstream, but BAX 499 has a longer half-life. When BAX 499 binds to TFPI, it allows the protein to persist for longer and, over time, to accumulate. And although the drug binds to TFPI, it does not completely deactivate it. So, as partially active TFPI piles up, the balance eventually tips from a pro-clotting effect to an anti-clotting effect. The problem seems to be confined to this particular compound, but nonetheless, the company has shifted its focus away from aptamers.
Baxter is now concentrating on peptides—short strings of amino acids that can be tailored to block part of the TFPI protein—a strategy that Scheiflinger and his colleagues first considered in 2005. The company has identified several promising candidates, but has not yet decided whether it will move them into clinical trials.
TFPI is not the only target for companies hoping to hamper the anticoagulant system. Alnylam Pharmaceuticals in Cambridge, Massachusetts, has set its sights on antithrombin—a protein produced by the liver that hinders clotting. “Antithrombin is probably one of the most potent natural anticoagulants we have in the body,” says Benny Sorensen, medical director of clinical research and development at Alnylam. But rather than inhibiting antithrombin's activity, the company plans to block its expression by using short strands of RNA to silence the messenger RNA that carries the code for antithrombin—an approach called RNA interference.
The company is testing its therapy, called ALN-AT3, in a safety study, and the initial results were presented at the World Federation of Haemophilia annual meeting in Melbourne, Australia, in May. After giving healthy volunteers a single low dose of the drug, expression of antithrombin was reduced by 28–32%—an outcome that Sorensen says left the researchers “very surprised”. They had thought that it would take higher doses to achieve such a result.
But Sorensen believes that they can do even better. In that first phase, the researchers were not allowed to exceed a 40% reduction in antithrombin because of the safety risks to healthy volunteers. The next phase of the study will include people with haemophilia, and there will not be the same limitation. So the researchers plan to administer multiple doses of the drug. Sorensen thinks that if they can achieve a 50–80% reduction in antithrombin, ALN-AT3 may be able to control bleeding in people with haemophilia without infusions of clotting factor.
Cautious optimism
All of these therapies have one major advantage over protein replacement: antibodies, peptides and RNA can be effective even when injected under the skin, in part because they are so much smaller than the proteins used for factor-replacement therapy. Novo Nordisk envisages putting its antibody into a ‘pen’ like the one that people with diabetes use to administer insulin. This would be much more convenient than the intravenous infusions required for existing therapies. “Haemophilia patients are pestered from when they are one or two years old for the rest of their lives with intravenous injections,” Sorensen says. “If we can achieve a correction of this haemostatic imbalance that would prevent spontaneous bleeds, then we've really offered an unbelievable change in the lives of these haemophilia patients.”
If compounds such as concizumab and ALN-AT3 prove effective, they will undoubtedly be a boon for at least one group of people with haemophilia: those who develop inhibitory antibodies against the blood-clotting factors VIII and IX, and who can no longer receive this standard therapy. Roughly 5% of those with haemophilia B fall into this category, and 30% of those with haemophilia A (see page S12). Baxter, Novo Nordisk and Alnylam think that their products will appeal to other people with haemophilia. But whether these therapies will be safe and effective enough to replace infusions of clotting factor “is the million-dollar question”, Scheiflinger says. Sorensen is the most optimistic. He speculates that a once-a-month dose of ALN-AT3 might control bleeding without the need for prophylactic infusions of clotting factor. Even if patients cannot completely forgo factor replacement, he adds, ALN-AT3 might allow them to use less, which could reduce the risk of developing inhibitors.
But many of the physicians who treat patients with haemophilia are not convinced. “The common thinking among haemophilia treaters is that these new strategies can never replace treatment with factor VIII and IX in non-inhibitor patients,” says Erik Berntorp, a haematologist at Lund University in Malmö, Sweden. David Ginsburg, a geneticist at the University of Michigan, Ann Arbor, is equally cautious. “In the case of a genetic deficiency, it's pretty hard to improve on replacing the missing factor,” he says.
Kenneth Mann, a biochemist at the University of Vermont in Burlington, does not doubt that blocking these anticoagulant pathways will increase the production of thrombin, a key protein in clotting, but he does not think that these therapies will necessarily work for everyone. People with haemophilia “are more heterogeneous than we'd like to admit,” he says. And companies will have to work out how to stratify patients on the basis of their real bleeding risk to determine who will benefit from these new approaches. “I don't mean to throw a wet blanket on this,” he says, “but caution is required.”
One risk is that these therapies will work too well, tipping the balance towards clotting. In a person without haemophilia, Ginsburg says, a total lack of antithrombin “seems to be disastrous”. Mice that lack either antithrombin or TFPI die in utero. Although the antithrombin-based therapies for haemophilia are not designed to completely block their targets, “knocking them down is not without risk”, he says. And as the failure of BAX 499 shows, the risks posed by any new medication can be hard to predict.
Jakob Back, vice-president of the concizumab project at Novo Nordisk, understands the scepticism. Protein replacement has been the go-to therapy for haemophilia for decades. Concizumab and similar therapies represent “a completely different way of approaching haemophilia compared to anything we've been doing for the last 50 years”, he says. “We are moving into unknown territory.”
This article is reproduced with permission and was first published on November 26, 2014
