Abstract
Objective:
To measure body mass index (BMI) and ambulation changes for a morbidly obese, 47-year-old man with chronic motor-incomplete tetraplegia after gastric sleeve surgery.
Design/Method:
A morbidly obese man, BMI=44 kg m−2, with chronic C5 AIS D tetraplegia underwent elective gastric sleeve surgery. Assessment of BMI and function via the 6-minute walk test (6MWT), 10-meter walk test (10MWT) and ambulation parameters (CIR Systems/GAITRite, Franklin, NJ, USA) was performed preoperatively and at 12, 24, 36 and 52 weeks postoperatively, and additionally after 3 weeks of both a prescribed coached (3 × /week facility based) and a non-coached (3 × /week home based) walking program initiated at 52 weeks. A step activity monitor assessed daily ambulation preoperatively, prior to and during the third and sixth week of the walking program.
Results:
Results included a 34.3% peak BMI decrease at 52 weeks post surgery and a peak increase in 6MWT distance of 58% at 52 weeks post surgery, 10MWT preferred speed of 56% at 55 weeks and step activity monitor of 82% at 58 weeks post surgery. At 58 weeks, gait data demonstrated a decrease in double limb stance of 38% and decrease in base of support of 72%.
Conclusion/Clinical Relevance:
This empirical case assessment of BMI and functional mobility before and after gastric sleeve surgery may encourage further investigation into mobility and general health effects post gastric procedures for people with chronic motor-incomplete spinal cord injury.
Similar content being viewed by others
Introduction
Approximately 240 000–337 000 Americans live with spinal cord injury/dysfunction (SCI/D) (https://www.nscisc.uab.edu/reports.aspx), with 40–60% experiencing secondary health risks of metabolic derangement and obesity,1 negatively impacting health-related quality of life and functional self-mobility. SCI/D is associated with decreased lean muscle mass and increased total body fat, notably visceral adiposity, which is a key precursor to the metabolic syndrome.1 Aerobic exercise and diet modification recommendations for weight management are an obvious solution; however, cardiovascular exercise presents obvious challenges for people with spinal cord injury, especially participation in moderate cardiovascular exercise.2, 3, 4 In addition, decreasing caloric intake may not result in the desired weight loss outcome for people with SCI/D, as muscle is infiltrated by adipose tissue, thereby reducing metabolic basal rate, which in turn facilitates weight gain and obesity.5 Inactivity prior to SCI, dependency upon powered mobility for safety and the SCI/D metabolic changes combine with reduced muscular function creating the potential for morbid obesity. Bariatric surgical procedures represent one option for morbidly obese people with SCI who fail to lose weight with more conservative measures.
Limited data exist describing bariatric surgery procedures for individuals with SCI/D; however, three case reports indicate positive metabolic and functional outcomes.6, 7, 8 Two non-ambulatory individuals demonstrated improvement in insulin resistance and dyslipidemia with medication discontinued, normalization of previously elevated fasting glucose, sleep hygiene no longer requiring bilevel positive airway pressure (BiPAP), and improved quality of life, energy level and execution of activities of daily living.6, 7 Wong et al.8 demonstrated a 58% improvement in 6-minute walk distance 7 months postoperatively in a person with chronic T12 AIS C paraplegia. Upon review of the bariatric literature, little emphasis on prescribed exercise is provided post surgery.9 Enabling individuals with SCI/D to exercise may be especially important as obesity, limited muscle function and anxiety to attempt increased mobility all co-exist. A ‘coached mobility’ model post bariatric surgery for individuals with SCI/D may optimally progress mobility device selection, distance, terrain variation and speed.
Case description
A 47-year-old man (RH) with C5 AIS D tetraplegia from a motor vehicle collision in 2005 and pre-injury obesity (~230 pounds) attempted unsuccessful weight reduction, including involvement in a 6-month exercise research protocol involving robotic locomotor training and aquatic therapy, as well as a faith-based and a nationally known weight loss program. RH chose to proceed with a gastric banding procedure at a hospital not affiliated with the rehabilitation center. At the time of procedure, he weighed 132.1 kg (290 pounds). No complications occurred during or after surgery. RH followed a strict prescribed diet regime and weekly diet mentoring sessions. He received absolutely no direction regarding physical activity or exercise.
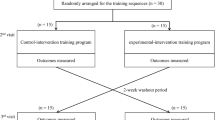
At baseline, RH used electric mobility, but he ambulated with a rolling walker for short, in-home distances. For testing purposes, all case report gait assessments were completed with two straight canes. Assessments were performed at baseline and then at 12, 24, 36 and 52 weeks post surgery, and after an additional 3 weeks each of coached (3 × /week in rehabilitation facility) and non-coached (3 × /week at home) walking program initiated at 52 weeks post surgery. The walking program consisted of supervised ambulation with a wheeled walker initially progressing to two forearm crutches. No specific directions were given regarding gait pattern. Instead, the walking program emphasized improving confidence over varying terrains such as grass, curbs and uneven sidewalks, and increasing ambulation distance. Outcome measures included BMI, 10-meter walk, 6-minute walk and gait assessment specifically evaluating symmetry and single limb stance time (Figure 1). In addition, a step activity monitor tracked the daily ambulation preoperatively, and in the third and sixth week of our coached mobility intervention.
Results
Body mass index
BMI decreased 15.1% at 12 weeks post surgery compared with baseline with additional but smaller reductions occurring at 24, 36 and 52 weeks post procedure. Overall, RH experienced a 34.3% reduction in BMI and a 40.1 kg (88 lbs) loss in body mass 52 weeks post surgery. The 6-week coached and non-coached walking intervention started at 52 weeks post surgery produced minimal additional BMI reduction.
10-meter walk
RH showed an ambulation speed of 0.44 m s−1 pre-surgery, 0.71 m sec−1 52 weeks post surgery and 0.79 m sec−1 after 3 weeks of coached walking. This represented a 57.4% improvement in the walking speed at 52 weeks post surgery and further improvement of 11.1% at week 55 after completion of the 3-week-coached walking intervention. However, his speed decreased at week 58 after 3 weeks of the non-coached walking program (−19.2%). Overall, RH progressed from home ambulatory (level 1–2) to community ambulatory with assistive device (level 4) status according to van Hedel.10
Six-minute walk
RH displayed large improvement in walking distance at 12 (15.1%) and 24 (34.4%) weeks post surgery. Total walking distance continued to increase at 36 and 52 weeks post surgery, with smaller changes of 8.0 and 3.1%. The 6-week walk intervention did not additionally improve total 6MWT distance, but the distance ambulated during each of the coached-walk intervention sessions increased to one half mile or 2640 from his initial 532 feet per session.
Step activity monitor and gait parameters
Total steps per day improved by 47% at 52 weeks post surgery with continued increase in step activity (21 and 13%) at 3 (coached) and 6 weeks (non-coached) during the walking intervention. Overall, RH displayed an 82% increase in steps per day. At 58 weeks, gait data demonstrated a decrease in double limb stance of 38% and a decrease in base of support of 72%. RH’s ambulation pattern displayed concurrent increases in smoothness and fluidity, and he demonstrated less dependence on power mobility and assistive devices (Figure 2). RH began the coached-walking program mainly using his powered scooter and ending the 6-week walking program independently ambulating into church, restaurants and social outings with a wheeled walker for long distances or two forearm crutches for shorter walks.
Discussion/Clinical Importance
RH experienced a 40.1 kg (88 lbs) loss in body mass at 52 weeks post gastric sleeve surgery, similar to other reported cases of individuals without spinal cord injury (http://www.nlm.nih.gov/medlineplus/ency/article/007199.htm).11 Over the same time period, his ambulatory ability improved considerably, aligning with the previous report of a 58% improvement in 6-minute walk distance 7 months post gastric procedure for an ambulatory individual with spinal cord injury.8 Improved cardio-metabolic and reported quality of life profiles also may occur with weight loss post bariatric procedures.11 We did not assess cardio-metabolic function, but our data reflect quality of life increases in step activity and less reported reliance on motorized mobility for outings to the mall, church and other social functions, as well as transitioning from wheelchair to bilateral forearm crutches as his primary mobility means. RH experienced an opportunity to increase his daily physical activity and alter his sedentary lifestyle, positively impacting his self-reported quality of life.
Consequently, additional weight loss may occur with greater total energy expenditure; hence, we implemented a 6-week-coached and non-coached walking program to not only increase daily energy expenditure with the goal of additional weight loss but also to empower RH to advance his ambulation status, taking advantage of his weight loss, which previously impeded his abilities. Our results do not support the utility of a coached and a non-coached walking program at 52–58 weeks post gastric sleeve procedure to promote further weight loss. The walking program, however, did produce increased gait speed and symmetry, community activity and distance walked.
Compliance may be a factor in this outcome as RH performed the initial 3 weeks of the walking program at our rehabilitation facility with research personnel providing guidance, support and supervision, and then completed the final 3 weeks of the walking program at home with minimal contact from research personnel (one contact day per week) with instructions to duplicate the frequency, intensity and duration of his home walking sessions with those of the first three coached walking weeks. We asked RH whether his home-based walking program matched the facility-based, supervised program. He reported duration, intensity and frequency inconsistencies during self-execution of the home-based walking program.
RH improved his ambulatory function with weight loss post gastric sleeve surgery without altering his physical activity, and as we anticipated the 6-week-coached and non-coached walking program, implemented 52 weeks post surgery, produced further functional improvement from household to community ambulator.10, 12 RH moved from 0.44 m s−1, ‘supervised walker with outdoor wheelchair dependency’, and approached ‘walker with aide’ at 0.79 m s−1, surpassing the minimally clinical important difference of 0.3 m s−1.10 The coached walking program provided RH a safety net to attempt both longer walk distances at faster speeds and to reduce assistive device base of support. Our experience with RH emphasizes the importance of ongoing supervision and guidance to achieve the optimum level of functional performance possible after gastric sleeve surgery for individuals with spinal cord injury.13, 14, 15, 16
References
Weaver F, Collins EG, Kurichi J, Miskevics S, Smith B, Rajan S et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil 2007; 86: 22–29.
Anderson J, Konz EC . Obesity and disease management: effects of weight loss on comorbid conditions. Obes Res 2001; 9: 326S–334S.
Jones G, Sinclair LB . Multiple health disparities among minority adults with mobility limitations: an application of the ICF framework and codes. Dis Rehabil 2008; 30: 901–915.
Pain H, Wiles R . The experience of being disabled and obese. Dis Rehabil 2006; 28: 1211–1220.
Gorgey AS, Mather KJ, Poarch HJ, Gater RJ . Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imaging. J Spinal Cord Med 2011; 34: 99–109.
Miyano G, Kalra M, Inge TH . Adolescent paraplegia, morbid obesity, and pickwickian syndrome: outcome of gastric bypass surgery. J Pediatr Surg 2009; 44: 41e–44e.
Alaedeen D, Jasper J . Gastric bypass surgery in a paraplegic morbidly obese patient. Obes Surg 2006; 16: 1107–1108.
Wong S, Barnes T, Coggrave M, Forbes A, Pounds-Cornish E, Appleton S et al. Morbid obesity after spinal cord injury: an ailment not to be treated? Eur J Clin Nutr 2013; 67: 998–999.
Egberts K, Brown WA, Brennan L, O’Brien PE . Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg 2012; 22: 335–341.
Van Hedel HJ . Gait speed in relation to categories of functional ambulation after spinal cord injury. Neurorehabil Neural Repair 2009; 23: 343–350.
Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013; 310: 2416–2425.
Bohannon RW, Glenney SS . Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract 2014; 20: 295–300.
Rimmer JH, Riley B, Wang E, Rauworth A, Jurkowski J . Physical activity participation among persons with disabilities: barriers and facilitators. Am J Prev 2006; 26: 419–425.
Scelza WM, Kalpakjian CZ, Zemper ED, Tate DG . Perceived barriers to exercise in people with spinal cord injury. Am J Phys Med Rehabil 2005; 84: 576–583.
Turk MA . Secondary conditions and disability. In: Field MJ, Jette AM, Martin L (eds). Workshop on Disability in America. National Academies Press: Washington, DC, 2006, pp 185–193.
Van der Ploe HP, Van Der Beek AJ, Van Der Woude LHV, van Mechelen W . Physical activity for people with a disability: a conceptual model. Sports Med 2004; 34: 639–649.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Perreault, J., Geigle, P., Gorman, P. et al. Improvement in weight loss and ambulation outcomes after gastric sleeve surgery for a person with chronic motor-incomplete tetraplegia: clinical case report. Spinal Cord 54, 750–753 (2016). https://doi.org/10.1038/sc.2016.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.22
This article is cited by
-
Recent Updates in Nutrition After Spinal Cord Injury: 2015 Through 2021
Current Physical Medicine and Rehabilitation Reports (2022)
-
Comparative Outcomes of Bariatric Surgery in Patients with Impaired Mobility and Ambulatory Population
Obesity Surgery (2018)