Abstract
Objectives:
To describe the presence of sperm in the prostatic urethra as a stand-alone ejaculation entity and to assess its characteristics.
Methods:
Thirty-three patients with anejaculation during sexual stimulation were enroled. Penile vibratory stimulations were carried out following bladder catherization and instillation of a pink buffering medium (Ferticult). Two-step catheterisation was then performed: a catheter was inserted through the urethral sphincter into the prostatic urethra to aspirate its content, and then bladder catheterisation was performed to collect the Ferticult. The procedure was repeated in some patients after at least 1 week. The characteristics of the semen collected from each organ were analysed and compared.
Results:
A total of 42 trials were obtained from 22 patients. Sperms were found in the prostatic urethra in 21 samples (50%) from 12 patients (11 with spinal cord injury, 1 with diabetes). The colour of all 21 prostatic urethra sperm samples differed from the Ferticult. Sperm motility was greater in 8 samples, sperm count was higher in 10 and pH was different in 10, compared with the bladder samples. The higher overall quality of the sperm allowed cryopreservation in 10 prostatic urethra samples compared with only 5 bladder samples. Four of the five patients who underwent repeated trials had a reproducible pattern of prostatic urethra ejaculation.
Conclusion:
The presence of sperm in the prostatic urethra most probably results from ‘ejaculation dyssynergia’, a lack of coordination between bladder neck and external sphincter. Sperm from the prostatic urethra should be systematically sought to improve the outcome of assisted reproduction.
Similar content being viewed by others
Introduction
Ejaculation constituted of two successive phases, emission and expulsion, which involves different pelvi-perineal anatomical structures.1, 2 Seminal emission involves the expulsion of accessory gland products and sperm into the posterior urethra, with closure of the bladder neck to prevent retrograde ejaculation. During projectile ejaculation, rhythmic involuntary contractions of the bulbocavernosus and ischiocavernosus muscles cause expulsion of the ejaculate from the distal urethra. Seminal emission is controlled by both sympathetic (seminal tract smooth muscle contraction) and parasympathetic (seminal fluid secretion) stimulation (with nerve fibres arising from T10 to L2 and from S2 to S4 spinal segments, respectively), whereas the projectile phase of ejaculation is controlled by somatic nerve fibres arising from S2 to S4.3
Ejaculation may be significantly impaired in men with spinal cord injury (SCI). Only 16% are able to ejaculate through sexual stimulation, and 52% require penile vibratory stimulation (PVS). Antegrade ejaculation (AE) occurs in most cases (65%), but a certain number of patients experience retrograde ejaculation (RE). RE may be pure (17 to 29%) or combined with AE (16%).4, 5 Although some RE sperm fractions from the bladder can be used for certain assisted reproductive techniques, the viability of sperm in retrograde samples is usually lower than in antegrade samples. Cryopreservation is thus not always possible with RE samples.6
Although the exact mechanism of RE is still unknown, there is some evidence that it may result from poor coordination between the external sphincter and the bladder neck during expulsion. This can prevent AE or may reduce semen volume.3, 7, 8 The hypothesis of ejaculatory dyssynergia is further supported by the fact that dyssynergic voiding also occurs in most patients with SCI. Coordination between detrusor contractions and relaxation of the external sphincter and/or or the bladder neck is poor, leading to absent or incomplete micturition.9, 10, 11
The aim of this study was to search for sperm in the prostatic urethra, using a new technique of prostatic catheterisation, which we developed, and to evaluate the characteristics of any sperm found. The presence of sperm in the prostatic urethra following PVS would demonstrate the existence of ejaculatory dyssynergia.8
Materials and methods
Patients
From November 2011 to December 2012, 104 consecutive patients with neurological disorders and ejaculatory dysfunction attended the Reproductive Medicine Centre (Toulouse). Of these, 33 who wished to father a child but had anejaculation during sexual intercourse or manual stimulation were enroled in the study.
All patients gave informed verbal consent for the procedure (that involved one additional catheterisation after PVS).
Procedure
All patients had undergone voiding cystourethrograms and urodynamic tests in the preceding months, as part of routine care. For the study, all patients underwent the usual procedure to investigate ejaculation. This involves catherization to completely empty all urine from the bladder, followed by the instillation of a pink buffering medium (40 ml of Ferticult, Fertipro NV, Belgium) and then PVS.5, 12 If they failed to ejaculate, PVS was combined with oral midodrine, starting with 5 mg and increased in steps of 7.5 mg until the patient ejaculated, up to 30 mg.
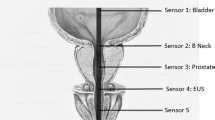
The urethra was then milked manually to ensure that as much semen as possible was collected. Following this, a two-step catheterization procedure was carried out (with two separate catheterizations): first, a catheter (Lofric Wellspect HealthCare, 12ch standard) was inserted through the urethral sphincter into the prostatic urethra, and a 5 ml syringe was used to aspirate the content of the prostatic urethra. Then, bladder catheterization was performed with a new catheter to collect the previously instilled Ferticult.
Several semen retrieval trials could be carried out in the same patient following an interval of at least 1 week. Cryopreservation was proposed to the patient if the quality of the sperm was sufficient, that is, when motile spermatozoa were present.
Biological analyses
Semen (that is, AE) analyses were performed according to the WHO recommendations.13
Bladder fraction (that is, RE)
After measuring the volume and testing the pH, 10 μl were examined between a slide and cover slide. When no sperms were found, the whole sample of urine was centrifuged at 400g (10 min). The pellet was then suspended in 1–2 ml of sperm-washing medium and analysed to confirm or infirm the absence of sperm. When sperms were present in the 10 μl or in the pellet, their characteristics were analysed.
Prostatic urethra ejaculation
Prostatic urethra ejaculation (PUE) is defined as the presence of sperm in the prostatic fluid aspirated from the prostatic urethra during the first catheterization. The prostatic urethra fraction was analysed in the same manner as the bladder fraction.
For each sample (AE, RE and PUE) obtained on a given day, several characteristics were analysed and recorded: colour, volume and pH of the liquid, as well as sperm count, progressive motility and viability. Total sperm count (TSC) in the antegrade fraction (AE) was calculated as sperm count × volume of antegrade specimen; TSC in the retrograde fraction (RE) was calculated as sperm count × volume of urine; TSC in the prostatic urethra fraction (PUE) was calculated as sperm count × volume of liquid.
To allow quantitative comparison of each characteristic of the RE and PUE fractions, the level of significance was set at >1.5 million for the sperm count, >4% for the progressive motility and >0.5 for the pH. The values for sperm count and motility corresponded to 10% of the lower reference limits recently published by the World Health Organization,13 whereas the value for pH was arbitrary.
Statistical analysis
The characteristics of the sperm from PUE and RE were compared as follows: descriptive variables were analysed by comparison of means (Wilcoxon signed rank test), and qualitative values were compared using χ2-tests. All statistical analyses were performed at the 5% level of significance using R 3.1.1 Development Core Team Software (R, Vienna, Austria).
Results
Ejaculation (that is, AE, RE or PUE, either pure or combined) was achieved in 22 out of 33 patients, and a total of 42 samples was collected. Patient characteristics are described in Table 1. Mean age was 29.0±9.1 years; mean time since the onset of the neurological disorder was 6.6±6.4 years. Nineteen patients had SCI, 2 had multiple sclerosis and 1 had diabetes. Eighteen out of the 19 patients with SCI had a complete motor lesion (ASIA Incapacity Scale A or B), and 1 had an incomplete motor lesion (ASIA Incapacity Scale C).
Nine patients took anticholinergics (trospium and/or oxybutynin) to treat detrusor overactivity and 17 took oral midodrine combined with PVS (mean 24.0±6.6 mg).
Prostatic urethra samples
Sperms were found in 21 prostatic urethra fractions (50%) (Table 2) from 12 patients (55%). Nine of these patients had upper motor neuron lesions, and 3 had lower motor neuron lesions; 11 patients were on midodrine, and 3 were on anticholinergics. There was a non-significant tendency for more prostatic urethra fractions in patients on midodrine (P=0,08) and less in those on anticholinergics (P=0,10). There was no effect of the level of the lesion (P=0,78).
Prostatic urethra vs bladder samples
All 21 PUE fractions were whitish in colour, whereas the bladder fractions were stained pink by the Ferticult. Compared with the bladder samples, sperm motility was higher in 8 PUE fractions and lower in 9 fractions; sperm count was higher in 10 PUE fractions (and lower in 10), and pH was different in 10 (out of 12 fractions) (Table 2). In one patient (no. 22), a PUE fraction was found with no AE or bladder RE fraction.
From a descriptive point of view, aside from the colour, 14 PUE fractions had at least 1 characteristic (sperm count, progressive motility or pH), which differed from the bladder fractions.
The quantitative values for bladder and PUE fractions are given in Table 3. Sperm count, motility, viability and pH values were all higher, although not significantly, in the PUE than the bladder fractions. TSC was significantly higher in the bladder.
PUE reproducibility
Repeat ejaculations were obtained in eight patients, at 3–8-week intervals. Three of these patients never had PUE. When a patient was found to have PUE, it was generally quite reproducible (Table 4): three patients consistently had PUE (100% of samples) and one frequently had PUE (80% of samples). In contrast, in patients who did not have PUE, the type of ejaculation was never constant in a given patient (except for one patient, no. 18).
PUE and type of ejaculation
PUE was associated with RE in 20 fractions: 12 times in pure RE (no AE) and 8 in mixed AE+RE ejaculation (Table 5). In one case (patient no. 22), PUE occurred with no concomitant AE or RE (Table 2). The type of ejaculation had no significant influence on the sperm characteristics of the PUE fractions (Table 5). For the bladder fractions, sperm counts and TSCs were significantly higher in the patients with pure RE.
Sperm cryopreservation
Cryopreservation was possible in 10 PUE fractions compared with 5 bladder fractions (P=0.11) (Table 2).
Discussion
This study demonstrated, for the first time, that it is possible to retrieve sperm from the prostatic urethra by direct catheterization. The presence of sperm in the prostatic urethra was not the result of urine contamination, as it was not stained by the Ferticult, and the sperm characteristics were of higher quality than those in the bladder RE. Equally, the PUE was not the result of sperm remaining in the urethra, as it could be found with no AE, and thorough urethral milking was always performed prior to the prostatic catheterization. Furthermore, sperm obtained by prostatic massage is known to be of poor quality,14, 15, 16 which was not the case of the sperm found in the prostatic urethra samples. PUE was reproducible in some patients and could occur without AE or RE.
PUE is thus quite distinct from the so-called RE, which occurs relatively frequently in men with SCI.4, 5 If AE does not occur, RE is defined as any sperm in the urine sample. If AE does occur, various definitions of RE have been proposed, based on the presence of sperm in the postejaculatory urine: sperm count above 5 million, 5–10% content of sperm or >5–10 sperm per high-power field.17, 18 These criteria describe the small quantity of semen that can be obtained in the urethra by catheterization after AE.5
RE occurs in different conditions in which the bladder neck is weakened or open. This usually arises when the sympathetic outflow to the bladder neck is reduced, such as in patients with a lower motor neuron lesion at the T10-L2 spinal segment (causing flaccidity)4, 5, 19 or those on alpha-blocking agents.20 RE has also been described as a side effect of intradetrusor botulinum toxin injection, possibly due to the spread of toxin to the bladder neck.21 However, RE also occurs in patients with upper motor neuron lesions and spasticity, who have a closed bladder neck, and thus other mechanisms must also be involved.5
Midodrine, an alpha1-adrenergic agonist, is a safe and efficient adjunct to PVS for anejaculation in patients with SCI. It stimulates seminal emission, most probably by a direct effect on the smooth muscles of the ejaculatory duct. Midodrine also increases bladder neck tone, which might explain the non-significant tendency to a higher rate of PUE in patients taking this drug. PUE was also more difficult to obtain in patients on anticholinergics; however, the influence of anticholinergics on ejaculation is yet to be studied in this population.
Recent studies have suggested a lack of coordination between the external sphincter and the bladder neck during expulsion as a possible cause of RE in patients with SCI. The simultaneous recording of external and internal sphincter pressures during PVS has demonstrated that different sphincteric events occur, depending on whether patients ejaculate or not.3 In successful PVS, external sphincter pressure first reaches a peak (average 180 cm H2O) that subsequently decreases and is followed seconds later by a peak in internal sphincter pressure (average 178 cm H2O), which exceeds external sphincter pressure, leading to ejaculation. In non-responders, external sphincter pressure never increases to more than 105 cm H2O.
Another study used colour Doppler ultrasound recordings of manually induced ejaculation in a healthy man and showed that the dilation of the prostatic urethra is immediately followed by a flattening of the bladder neck and contraction of the prostate, creating a pressure chamber that precedes the expulsion of semen by a few seconds. In a patient with SCI and RE, the prostatic urethra dilated, but activity of the prostate and bladder neck was less marked. The external sphincter never relaxed, preventing AE, whereas the bladder neck first contracted then relaxed a few seconds later, allowing the semen remaining in the prostatic urethra to slowly flow into the bladder.7
To elucidate the mechanisms behind the presence of sperm in the prostatic urethra, further studies on the dynamics of ejaculation involving pressure recordings or Doppler ultrasonography, followed by prostatic catheterization, need to be carried out.
This lack of coordination during ejaculation appears to be very similar to the sphincter dyssynergia found during bladder contraction in patients with upper motor neuron lesions following SCI.8 Dyssynergia of the external detrusor sphincter is very commonly associated with neurogenic detrusor overactivity.9, 10 Contraction of the bladder neck during detrusor contraction is another entity, characteristic of sympathetic overactivity below the level of the lesion.11 Although detrusor sphincter dyssynergia is most commonly found and treated, both types of dyssynergia can cause incomplete voiding or difficulty with catheterisation.
Conclusion
We demonstrated that sperm may be present in the prostatic urethra, independently from the occurrence of either AE or RE. The systematic collection of this sperm, which is more often of higher quality than that found in the bladder, should improve the outcome of assisted reproduction.
We therefore recommend a change in practice towards a two-step catheterisation procedure, involving extraction of the prostatic urethra content first. Further studies should seek to develop and standardise this technique of prostatic urethra catheterisation. In particular, the conception of a new type of catheter that could be used to empty both the lumen and the prostatic ureter in a single procedure (which was not possible with our catheter) would simplify the technique.
DATA ARCHIVING
There were no data to deposit.
References
Biering-Sorensen F, Sonksen J . Sexual function in spinal cord lesioned men. Spinal Cord 2001; 39: 455–470.
Chehensse C, Bahrami S, Denys P, Clement P, Bernabe J, Giuliano F . The spinal control of ejaculation revisited: a systematic review and meta-analysis of anejaculation in spinal cord injured patients. Hum Reprod Update 2013; 19: 507–526.
Sonksen J, Ohl DA, Wedemeyer G . Sphincteric events during penile vibratory ejaculation and electroejaculation in men with spinal cord injuries. J Urol 2001; 165: 426–429.
Brackett NL, Ferrell SM, Aballa TC, Amador MJ, Padron OF, Sonksen J et al. An analysis of 653 trials of penile vibratory stimulation in men with spinal cord injury. J Urol 1998; 159: 1931–1934.
Soler JM, Previnaire JG, Plante P, Denys P, Chartier-Kastler E . Midodrine improves ejaculation in spinal cord injured men. J Urol 2007; 178: 2082–2086.
Brackett NL, Bloch WE, Lynne CM . Predictors of necrospermia in men with spinal cord injury. J Urol 1998; 159: 844–847.
Nagai A, Watanabe M, Nasu Y, Iguchi H, Kusumi N, Kumon H . Analysis of human ejaculation using color Doppler ultrasonography: a comparison between antegrade and retrograde ejaculation. Urology 2005; 65: 365–368.
Soler JM, Previnaire JG . Ejaculatory dysfunction in spinal cord injury men is suggestive of dyssynergic ejaculation. Eur J Phys Rehabil Med 2011; 47: 677–681.
Blaivas JG . The neurophysiology of micturition: a clinical study of 550 patients. J Urol 1982; 127: 958–963.
Karsenty G, Reitz A, Wefer B, Boy S, Schurch B . Understanding detrusor sphincter dyssynergia—significance of chronology. Urology 2005; 66: 763–768.
Schurch B, Yasuda K, Rossier AB . Detrusor bladder neck dyssynergia revisited. J Urol 1994; 152: 2066–2070.
Soler JM, Previnaire JG, Plante P, Denys P, Chartier-Kastler E . Midodrine improves orgasm in spinal cord-injured men: the effects of autonomic stimulation. J Sex Med 2008; 5: 2935–2941.
World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn, WHO Press: Geneva, Switzerland. 2010.
Arafa MM, Zohdy WA, Shamloul R . Prostatic massage: a simple method of semen retrieval in men with spinal cord injury. Int J Androl 2007; 30: 170–173.
Brackett NL . Infertility in men with spinal cord injury: research and treatment. Scientifica (Cairo) 2012; 2012: 578257.
Momen MN, Fahmy I, Amer M, Arafa M, Zohdy W, Naser TA . Semen parameters in men with spinal cord injury: changes and aetiology. Asian J Androl 2007; 9: 684–689.
Okada H, Fujioka H, Tatsumi N, Kanzaki M, Inaba Y, Fujisawa M et al. Treatment of patients with retrograde ejaculation in the era of modern assisted reproduction technology. J Urol 1998; 159: 848–850.
Kendirci M . Retrograde ejaculation: etiology, diagnosis, and management. Curr Sex Health Rep 2006; 3: 133–138.
Rodic B, Curt A, Dietz V, Schurch B . Bladder neck incompetence in patients with spinal cord injury: significance of sympathetic skin response. J Urol 2000; 163: 1223–1227.
Hellstrom WJ, Sikka SC . Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol 2006; 176: 1529–1533.
Caremel R, Courtois F, Charvier K, Ruffion A, Journel NM . Side effects of intradetrusor botulinum toxin injections on ejaculation and fertility in men with spinal cord injury: preliminary findings. BJU Int 2011; 109: 1698–1702.
Acknowledgements
We are grateful to Dr Vincke Bernard for statistical assistance, and to Johanna Robertson for revision of the English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Soler, JM., Previnaire, J. & Mieusset, R. Evidence of a new pattern of ejaculation in men with spinal cord injury: ejaculation dyssynergia and implications for fertility. Spinal Cord 54, 1210–1214 (2016). https://doi.org/10.1038/sc.2016.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.78
This article is cited by
-
Urethral pressure profile during ejaculation in men with spinal cord injury
International Journal of Impotence Research (2023)
-
Treatment of Delayed Ejaculation
Current Sexual Health Reports (2020)
-
Fertility and sexuality in the spinal cord injury patient
World Journal of Urology (2018)