Abstract
Objectives:
We investigated the ability of intraoperative neurophysiological monitoring to predict postoperative neurological recovery in intradural–extramedullary spinal cord tumors.
Methods:
From 2010 to 2014, we operated on 173 intradural–extramedullary spinal cord tumor patients with intraoperative neurophysiological monitoring. We retrospectively compared preoperative and postoperative clinical status using a modified McCormick grading scale and correlated with intraoperative neurophysiological monitoring. We followed patients for at least 1 year and correlated neurological outcomes with intraoperative changes in intraoperative neurophysiological monitoring. We then compared the degree of intraoperative neurophysiological monitoring change with the duration of the neurological deficit.
Results:
Monitorability was 92% and 57% with transcranial motor-evoked potential and somatosensory-evoked potential modalities, respectively. Waveform attenuation on transcranial motor-evoked potentials was detected in 8.17% of cases. For somatosensory-evoked potentials, waveform attenuation was detected in 7% of the patients. A multimodality approach incorporating any transcranial motor-evoked potential changes had a sensitivity of 0.91 and a specificity of 0.98. The McCormick grade scale increased until 1 month in patients with alarm criteria on transcranial motor-evoked potentials (P<0.05).
Conclusions:
Patients suffered neurological deterioration in case of abolishment or >50% irreversible attenuation of the waveform in transcranial motor-evoked potentials. All patients gradually recovered after 1 postoperative month with alarm criteria from 50% to 80% irreversible amplitude drop on transcranial motor-evoked potentials.
Similar content being viewed by others
Introduction
Neurosurgical excision of space-occupying lesions within or in the vicinity of the spinal cord poses significant risk to postoperative neurological outcomes. Significant neurological sequelae are infrequent, but potentially disastrous consequences can occur during such procedures.1 To address this risk, application of intraoperative neurophysiological monitoring (IONM) via assessment of somatosensory-evoked potentials (SEPs), transcranial motor-evoked potentials (tcMEPs) and electromyography of relevant nerve root myotomes to detect impending neurological injury has gained acceptance during recent decades.2, 3
Despite the significant advances in IONM techniques and applications, there are no data on the long-term outcomes and recovery in patients with alarm criteria corresponding to an aggravated postoperative neurological status. Accordingly, this study retrospectively analyzed the correlations between the alarm criteria in IONM and the duration of the neurological deterioration and recovery in patients who underwent intradural–extramedullary spinal cord tumor surgery. The aim of this study was to explore the ability of IONM to predict the postoperative neurological recovery.
Materials and methods
Patients
A total of 173 consecutive patients (65 men, 108 women; mean age 49.1 years, range 14–81 years) who underwent intradural–extramedullary spinal cord tumor surgery with IONM at Asan Medical Center, Seoul, Korea from January 2010 to July 2014 were identified from a digital database. All operations were performed by a senior neurosurgeon (SCR) with IONM monitoring. We followed the patients for at least 1 year and correlated patient neurological outcomes with intraoperative changes in the IONM. We then compared the degree of IONM change with the duration of the neurological deficit. We also analyzed the degree of tumor resection, tumor pathology and tumor location. We excluded patients with recurrent tumors.
Neurological assessment
The neurological state of each patient was evaluated before surgery, immediately after surgery, at 1, 6 and 12 months later and then once per year thereafter. The clinical status was established according to a modified version of the McCormick grading scale, which is a functional scale designed to account for both motor and sensory functions (Table 1).4 As the McCormick grading scale is hard to differentiate between radiculopathy and myelopathy, in our study we classified patients with myelopathy.
Neurophysiological monitoring
Intraoperative SEPs
SEP stimulation was accomplished with square wave electrical pulses of 0.3-ms duration and a maximum intensity of 25 mA at a frequency of 5 Hz. Surface-stimulating electrodes were located over each median nerve at the wrist and over each posterior tibial nerve at the ankle. Evoked potentials were recorded in a referential manner from the C3 (right median nerve stimulation), C4 (left median nerve stimulation) and Cz (right and left tibial nerve stimulation) positions and from a reference electrode at FPZ (International 10–20 system).
Intraoperative tcMEPs
Multipulse transcranial electrical stimulation was carried out using a commercially applicable IONM electrical stimulator (Neuropack MEB-9200 K; Nihon Kohden Co., Tokyo, Japan). Nine-millimeter disc electrodes were fixed to the scalp with collodion 6 cm anterior to Cz and at C3 and C4 (International 10–20 system). Trains of either four or five pulses (individual stimulus duration, 50 ms) with interstimulus intermissions of 2, 3 or 4 ms were used, depending on which provided the best recording, with a period of at least 30 s between two consecutive trains. Stimulus intensity was gradually increased (50-V augmentations from 100 V to a maximum of 600 V) until tcMEP amplitudes were maximized above a minimum of 20 mV. If response amplitudes of at least 20 mV could not be obtained from either leg, tcMEPs monitoring was abandoned.
TcMEPs were recorded concurrently from the abductor pollicis brevis muscles of both arms and from the tibialis anterior and abductor hallucis muscles of both legs using a pair of non-insulated subcutaneous needle electrodes inserted 3 cm apart in each muscle. The time base was 100 ms, and the filter band pass was 20–5000 Hz, using restricted high-pass filters.
Electrophysiological monitoring
IONM was performed throughout surgery. Baseline readings were obtained before the skin incision and after opening of the lamina. Waveforms were evaluated for latency and peak-to-peak amplitude. Stimulation was alternated between SEPs and tcMEPs in a continuous order. SEPs amplitude reductions of >50% of baseline values and latency increases >10% were regarded as significant. During intravenous maintenance anesthesia, tcMEP amplitude diminutions >50% of baseline values were contemplated indicative of a significant change, providing that the levels of neuromuscular blockade and general anesthesia were unchanged. Compound muscle action potentials were recorded either before or after each tcMEP trial. The surgical team was immediately notified of any significant IONM changes.
Alarm criteria and corresponding surgical parameters
The neurological alarm criteria in tcMEPs were abolishment or >50% irreversible attenuation of the waveform, whereas in SEPs they were >50% amplitude attenuation and/or prolongation of latency >10% (Table 2).1 If any significant changes in tcMEPs and SEPs occurred, the surgical procedure was stopped temporarily. We then checked the mean blood pressure and increased the blood pressure to at least 60 mm Hg. In addition, we irrigated the surgical field using warm saline solution. We monitored tcMEP and SEP changes regularly, particularly during the following steps: (1) patient positioning, (2) laminectomy, (3) dura opening, (4) tumor resection, (5) dura closure, and (6) after laminar insertion. In addition, we performed tcMEPs whenever the surgeon checked for spinal cord injury.
Anesthesia management
The anesthetic protocol used during surgery contained a combination of two drugs, remifentanil (0.25 mg kg−1 min−1) and propofol (10 mg kg−1 h−1), with total intravenous anesthesia. A single bolus of a non-depolarizing short-acting muscle relaxant (rocuronium) was given at induction to ease tracheal intubation and ventilation. Induction was obtained with a continuous infusion of remifentanil at 0.10–0.20 mg kg−1 min−1 and maintained with 0.25–0.40 mg kg−1 min−1. Target-controlled infusion was used for propofol with a plasma concentration for induction of 3–4 mg ml−1 and maintenance with 4–5 mg ml−1. No muscle relaxants were used after induction and intubation. The level of neuromuscular block was monitored by recording the compound muscle action potentials to a train of four stimuli. Electrocardiogram, invasive blood pressure, pulse oximetry, end-tidal carbon dioxide concentration and temperature were monitored.
Statistical analysis
Patient data were analyzed to identify any statistical associations between tcMEPs/SEPs and postoperative outcomes, with consideration of age, gender, tumor pathology, tumor location and extent of tumor removal. The demographic characteristics of the two groups were compared using independent t-tests to compare mean values. The chi-square test was used to compare proportional data, and linear mixed-effect models were used to investigate the geographic prediction of recovery. SPSS 17.0 for windows (SPSS, Chicago, IL, USA) was used for data entry and analysis, and a P-value <0.05 was considered statistically significant.
Results
Pathology, tumor location and removal
Surgeries were performed for resection of schwannomas in 133 patients (76.8%), meningiomas in 31 (18%) and ependymomas in 9 (5.2%). Tumor locations were cervical in 48 patients (27.7%), thoracic in 45 (26%), thoracolumbar in 12 (6.9%), lumbar in 65 (37.5%) and sacral in 3 (1.9%). Gross total resection was performed in 154 patients (89%). There were no statistically significant correlations between IONM and the degree of tumor resection, tumor location and tumor pathology.
Monitorability
Monitorability was 92% and 57% with tcMEP and SEP modalities, respectively. tcMEPs were monitorable in 159 patients and SEPs were performed in 99 cases. Waveform attenuation on tcMEPs was detected in 8.17% of patients. There were 10 true-positive outcomes (Table 3). For SEPs, waveform attenuation was detected in 7% of cases. However, there were no true-positive results. Only one was false-negative result with tcMEP/SEP monitoring. The patient who was diagnosed with L1 meningioma had no changes on tcMEP/SEP monitoring. After surgery, myelopathy occurred and required cane for ambulation. Symptoms recovered after 1 postoperative month. A multimodality approach incorporating any change in tcMEPs had a sensitivity of 0.71 and a specificity of 0.98 (Table 4).
Neurophysiological criteria and postoperative clinical correlations
When the amplitude of the tcMEPs and SEPs increased, the McCormick grade scale improved or was unchanged in the immediate postoperative period compared with the preoperative condition. However, this difference was not statistically significant (P>0.05). Most reports of tcMEP monitoring during surgery use the amplitude drop, usually from 50% to 80% of the baseline, as the primary warning criterion.5, 6, 7, 8, 9 Therefore, we compared patients with an amplitude that was 50%, 80% of the baseline values and yes/no warning criteria. There were no statistically significant differences in neurological deficits between patients with those different alarm criteria. We then set the tcMEP warnings as >50% irreversible attenuation of the waveform (Figure 1).
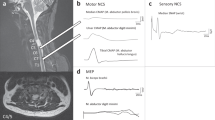
Tracing of SEPs (a) and tcMEPs (b) during the critical stage of the operation in patient no. 10. Right, TA and AH alarm criteria of tcMEPs with electromyography neurotonic discharge without SEP change. A transient motor deficit in the right leg occurred postoperatively. (c) T1-weighted gadolinium-enhanced MRI showing L3 schwannoma. (d) Intraoperative image of tumor removal. AH, abductor hallucis muscle; APB, abductor pollicis brevis; PT, posterior tibial nerve; TA, tibialis anterior. A full color version of this figure is available at the Spinal Cord journal online.
We divided the patients into two groups—tcMEPs and SEPs—to analyze the neurological recovery period after the operation in IONM alarm criteria patients and analyzed the correlation between intraoperative tcMEP/SEP warnings and the postoperative neurological outcome (Table 5). We used a linear mixed-effect model to investigate the geographic prediction of recovery. The McCormick grade scale of SEPs and tcMEPs showed recovery, as predicted by linear mixed-effect modeling, but this result was not statistically significant (P>0.05).
Of the 159 patients in our current series who underwent tcMEPs, 10 (6.2%) showed alarm criteria and neurological deterioration (Table 3). In these patients, the postoperative McCormick grade scale increase was statistically significant until 1 month (P<0.05; Figure 2). However, aspects of recovery after 1 month were different. In two cases of >80% irreversible attenuation of waverform in tcMEPs, the McCormick grade scale did not recover until 1 year. However, the McCormick grade scale subsequently gradually decreased in follow-up in cases of 50–80% irreversible amplitude drop. However, there were no correlations between SEP warnings and clinical outcome (P>0.05).
Linear mixed-effect models for the McCormick score over time. The McCormick grade scale for tcMEPs and SEPs showed recovery, as predicted by linear mixed-effect modeling, but the result was not statistically significant (P>0.05). (a) In tcMEPs, there was a correlation between alarm criteria and neurological deterioration. In these patients, the postoperative McCormick grade increase was statistically significant until 1 month (P<0.05). (b) In SEPs, the McCormick grade scale gradually decreased in follow-up, regardless of the degree of IONM change. However, there was no correlation between an SEP warning and clinical outcome (P>0.05). A full color version of this figure is available at the Spinal Cord journal online.
Representative case
A 67-year-old female patient presented at the Asan Medical Center outpatient clinic with right leg pain and bladder dysfunction (Table 3, patient 9). Her neurological examination was otherwise unremarkable. Magnetic resonance imaging of the spinal cord showed a 3.6-cm long peripheral enhanced cystic mass of the cauda equina at the L3-4 level (Figure 3c). The patient underwent tumor resection. Although SEPs were stable, tcMEPs deteriorated during tumor resection (Figures 3a and b), leading to the operation being temporarily stopped for correction of blood pressure and body temperature and for warm saline irrigation. However, tcMEP of the left abductor pollicis muscle decreased, with 72% irreversible attenuation of the waveform (Figure 3b). Immediate postoperative neurological examination revealed mild motor weakness (grade 4) and was associated with severe left leg pain.
Tracing of SEPs (a) and tcMEPs (b) during the critical stage of the operation in patient no. 9. Left, APB and TA alarm criteria of tcMEPs with electromyography neurotonic discharge without SEP change. A transient sensory and motor deficit in the left leg occurred postoperatively. (c) Preoperative T1-weighted gadolinium-enhanced MRI showing L3-4 schwannoma. (d) Postoperative T1-weighted gadolinium-enhanced MRI showing gross total removal of the tumor. AH, abductor hallucis muscle; APB, abductor pollicis brevis; PT, posterior tibial nerve; TA, tibialis anterior. A full color version of this figure is available at the Spinal Cord journal online.
Postoperative MRI confirmed gross total removal of the tumor (Figure 3d), which was diagnosed as schwannoma on histopathological examination. The patient’s postoperative neurological status did not change, and she was discharged on postoperative day 7. Follow-up examination at postoperative month 1 showed a decrease in pain and resolution of motor weakness (grade 5). During visits at postoperative months 6 and 12, the patient required minimal narcotic pain medication and could fully ambulate independently.
Discussion
Correlations between IONM changes and the clinical outcomes provide clear evidence in support of the logical assumption that surgical feedback showing evidence of electrical changes in evoked potentials may contribute to the preservation of a patient’s neurological status. With the advent of routine multimodal IONM of evoked potentials, a wide range of spinal surgical procedures that are associated with significant neuronal risk have been performed under electrophysiological supervision. IONM in the context of intradural–extramedullary spinal cord tumor surgery is feasible and valid. It may help identify neural injury at initial reversible stages and, when addressed appropriately, may spare untoward deficits.1
The first formal historically controlled report presenting an improvement in neural outcome due to IONM-monitored intramedullary spinal cord tumor removal described a mean 0.28 increase in the McCormick functional grade, in contrast to a mean 0.16 decrease among unmonitored patients.10 Furthermore, the intraoperative recovery of tcMEPs can indicate a postoperative improvement in motor function.11 However, once neurological deterioration occurs, it lasts for several months, and the neurological handicap represents a significant burden.
In some contexts, the integration of intraoperative monitoring in spinal surgery has been shown to help enhance the assessment of neural integrity and to guide intraoperative decision-making by predicting neurological outcomes, enabling the relevant preventive measures to be taken.12 Furthermore, some studies have shown that IONM predicts postoperative clinical prognosis. In one such study conducted in patients with compression of the spinal cord or cauda equina that resulted in low or absent tcMEPs at the start of surgery, an increase in potentials after surgical decompression could predict clinical function recovery.11 Therefore, IONM should not only focus on decreases in motor potentials but also consider the recovery of tcMEPs of muscle groups that are unresponsive before surgery.11
An earlier case report has described the intraoperative recovery of tcMEPs together with an improvement in neurological function after surgical decompression.13 In our experience, when the intraoperative amplitudes of tcMEPs and SEPs are increased, the McCormick grade scales improved or were unchanged in the immediate postoperative period compared with the preoperative condition. However, this difference was not statistically significant (P>0.05).
The basic mechanism of muscle tcMEPs generation is temporal and involves spatial summation of lower motor neuron excitatory postsynaptic potentials.14 Motor units exhibit on–off behavior: excitatory postsynaptic potential summation reaching or exceeding the firing threshold produces a full response, whereas anything less produces no response.15 Muscle tcMEPs show more graduated modulation as individual units add to or drop out of the compound potential. Nevertheless, being built from motor units, they are still non-linear, so that a disproportionately large reduction can follow a small decrease in corticospinal drive or lower motor neuron excitability.15, 16 This high sensitivity makes muscle tcMEP deterioration an imperfect predictor of motor deficit severity or permanence. On the other hand, tcMEP preservation provides good evidence for central motor pathway integrity. This physiology explains the postoperative improvement in motor function when intraoperative recovery of the tcMEPs is detected.17
Our current study is the first to predict the neurological recovery period from IONM findings after a intradural–extramedullary spinal cord tumor operation. Our results suggest that IONM is not only useful to decrease the incidence of postoperative neurological worsening but also to predict the neurological recovery.
According to our present findings, patients with alarm criteria on their tcMEP neurological status showed postoperative deterioration for 1 month. However, in cases of 50–80% irreversible attenuation of waveform in tcMEPs gradually recovered 1 month later. We investigated the mechanisms of clinical recovery after tcMEP decrease. TcMEP amplitudes seem to allow an objective assessment of the severity of corticospinal damage and thus enable a prediction of the recovery of function mediated by the corticospinal tracts. We can interpret the amplitude drop of tcMEPs during spinal cord operations as spinal cord injury. The patients in our present study with intradural–extramedullary tumors did not undergo myelotomy; hence, we can interpret the decrease in tcMEPs as a transient corticospinal tract injury. The cause of the transient spinal cord injury may be an intraoperative contusion or manipulation, as well as edema or hemodynamic change. Nerve root injury can be excluded by using free-running electromyography. These transient spinal cord injuries recover over time. A gradual strengthening of synaptic interconnections between distant spinal segments during several months after spinal cord injury has been described.18 Some fiber remyelination of damaged spinal tract fibers could also be responsible.19 Neurological recovery takes <3 months after incomplete spinal cord injury with spine trauma.20
Independent of clinical measures, clinical outcomes and walking function after a spinal cord injury can be predicted by the assessment of tcMEPs.21
This study had some limitations of note. First, IONM is helpful for intradural–extramedullary spinal cord tumors but with a lower sensitivity than for intradural–intramedullary spinal cord tumors. A second factor was the small number of patients with alarm criteria indicating neurological deterioration. Although greater numbers of patients need to be evaluated in the future to derive statistically meaningful conclusions, our study is the first to have been conducted in this area.
Conclusion
Our present study provides the first information on the prediction of neurological recovery when there is a tcMEP decrease during intradural–extramedullary spinal cord surgery. IONM is feasible and useful in the context of intradural–extramedullary spinal cord surgery for identifying iatrogenic injury to the spinal cord and predicting patient prognosis. Patients suffered neurological deterioration in case of abolishment or >50% irreversible attenuation of the waveform in tcMEPs. All patients gradually recovered after 1 postoperative month with alarm criteria from 50% to 80% irreversible amplitude drop on tcMEPs. We suggest that an amplitude drop of >80% should be prevented, whereas a drop between 50% and 80% may still be associated with postoperative neurological recovery. Although this description of a cohort of intradural–extramedullary spinal cord surgery may show that neurophysiological and clinical recovery over time are related, the underlying mechanisms of this remain unknown. Further analysis must be aimed at understanding the restorative mechanisms in the human T spinal cord.
Data archiving
There were no data to deposit.
References
Korn A, Halevi D, Lidar Z, Biron T, Ekstein P, Constantini S . Intraoperative neurophysiological monitoring during resection of intradural extramedullary spinal cord tumors: experience with 100 cases. Acta Neurochir (Wien) 2015; 157: 819–830.
Sutter M, Deletis V, Dvorak J, Eggspuehler A, Grob D, Macdonald D et al. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J 2007; 16 (Suppl 2): S232–S237.
Tamaki T, Kubota S . History of the development of intraoperative spinal cord monitoring. Eur Spine J 2007; 16 (Suppl 2): S140–S146.
Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ . Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg 2000; 93: 183–193.
Meylaerts SA, Jacobs MJ, van Iterson V, De Haan P, Kalkman CJ . Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg 1999; 230: 742–749.
Kombos T, Suess O, Ciklatekerlio O, Brock M . Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex. J Neurosurg 2001; 95: 608–614.
Bartley K, Woodforth IJ, Stephen JP, Burke D . Corticospinal volleys and compound muscle action potentials produced by repetitive transcranial stimulation during spinal surgery. Clin Neurophysiol 2002; 113: 78–90.
Langeloo DD, Lelivelt A, Louis Journee H, Slappendel R, de Kleuver M . Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa 1976) 2003; 28: 1043–1050.
Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ . Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am 2004; 86-a: 1248–1253.
Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery 2006; 58: 1129–1143.
Visser J, Verra WC, Kuijlen JM, Horsting PP, Journee HL . Recovery of TES-MEPs during surgical decompression of the spine: a case series of eight patients. J Clin Neurophysiol 2014; 31: 568–574.
Morota N, Deletis V, Constantini S, Kofler M, Cohen H, Epstein FJ . The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 1997; 41: 1327–1336.
Barley JL, Mooney JF, Glazier SS, Johnson T, Kornegay AL, Turner RP et al. Sudden appearance of new upper extremity motor function while performing neurophysiologic intraoperative monitoring during tethered cord release: a case report. J Pediatr Orthop 2010; 30: 624–628.
Taylor BA, Fennelly ME, Taylor A, Farrell J . Temporal summation—the key to motor evoked potential spinal cord monitoring in humans. J Neurol Neurosurg Psychiatry 1993; 56: 104–106.
Amassian VE. Animal and human motor system neurophysiology related to intraoperative monitoring. In: Deletis V, Shils JL (eds). Neurophysiology in Neurosurgery: A Modern Intraoperative Approach, 1st edn. Academic Press: San Diego, CA, USA, 2002, pp 3–23.
Macdonald DB . Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput 2006; 20: 347–377.
Macdonald DB, Skinne S, Shils J, Yingling C . Intraoperative motor evoked potential monitoring - a position statement by the American society of neurophysiological monitoring. Clin Neurophysiol 2013; 124: 2291–2316.
Calancie B, Alexeeva N, Broton JG, Molano MR . Interlimb reflex activity after spinal cord injury in man: strengthening response patterns are consistent with ongoing synaptic plasticity. Clin Neurophysiol 2005; 116: 75–86.
Curt A, Van Hedel HJ, Klaus D, Dietz V . Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma 2008; 25: 677–685.
Geisler FH, Dorsey FC, Coleman WP . Recovery of motor function after spinal-cord injury—a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med 1991; 324: 1829–1838.
Petersen JA, Spiess M, Curt A, Dietz V, Schubert M . Spinal cord injury: one-year evolution of motor-evoked potentials and recovery of leg motor function in 255 patients. Neurorehabil Neural Repair 2012; 26: 939–948.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rho, Y., Rhim, S. & Kang, J. Is intraoperative neurophysiological monitoring valuable predicting postoperative neurological recovery?. Spinal Cord 54, 1121–1126 (2016). https://doi.org/10.1038/sc.2016.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.65
This article is cited by
-
Wireless facial biosensing system for monitoring facial palsy with flexible microneedle electrode arrays
npj Digital Medicine (2024)
-
Spinal intradural extramedullary tumors: microscopic keyhole resection with the focus on intraoperative neurophysiological monitoring and long-term outcome
Journal of Orthopaedic Surgery and Research (2023)
-
Soft and stretchable organic bioelectronics for continuous intraoperative neurophysiological monitoring during microsurgery
Nature Biomedical Engineering (2023)
-
The role of intraoperative extensor digitorum brevis muscle MEPs in spinal surgery
European Spine Journal (2023)