Abstract
Objectives:
Spinal cord injury (SCI) often results in severe dysfunction of the autonomic nervous system. C1-C8 SCI affects the supraspinal control to the heart, T1-T5 SCI affects the spinal sympathetic outflow to the heart, and T6-T12 SCI leaves sympathetic control to the heart intact. Heart rate variability (HRV) analysis can serve as a surrogate measure of autonomic regulation. The aim of this study was to investigate changes in HRV patterns and alterations in patients with acute traumatic SCI.
Methods:
As soon as possible after SCI patients who met the inclusion criteria had 24 h Holter monitoring of their cardiac rhythm, additional Holter monitoring were performed 1, 2, 3 and 4 weeks after SCI.
Results:
Fifty SCI patients were included. A significant increase in standard deviation of the average normal-to-normal (SDANN) sinus intervals was seen in the first month after injury (P=0.008). The increase was only significant in C1-T5 incomplete patients and in patients who did not experience one or more episodes of cardiac arrest. Significant lower values of Low Frequency Power, Total Power and the Low Frequency over High Frequency ratio were seen in the C1-T5 SCI patients compared with T6-T12 SCI patients.
Conclusions:
The rise in SDANN in the incomplete C1-T5 patients could be due to spontaneous functional recovery caused by synaptic plasticity or remodelling of damaged axons. That the autonomic nervous system function differs between C1-C8, T1-T5 and T6-T12 patients suggest that the sympathovagal balance in both the C1-C8 and T1-T5 SCI patients has yet to be reached.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) patients with tetraplegia and high level paraplegia are known to suffer from dysfunction of the autonomic nervous system (ANS) including the autonomic regulation of the heart.1
The severity and the neurological level of the SCI have major impact on ANS function.2 Patients with SCI above T1 often have intact efferent vagal and sympathetic neural pathways innervating the heart. However, they are deprived of the supraspinal control causing reduced sympathetic activity below the level of SCI. Furthermore, the loss of supraspinal control causes morphological changes in sympathetic preganglionic neurons and peripheral alpha-adrenoceptor hyperresponsiveness.1 Patients with T1-T5 SCI lose cardiac sympathetic preganglionic neurons as the injury occurs corresponding to the sympathetic outflow to the heart, and likewise the postganglionic sympathetic innervation to the heart undergoes plastic changes.3 Individuals with SCI at T6 and below have intact cardiac spinal sympathetic neurons and intact innervation of the heart.
In tetraplegic individuals with complete lesions, the disconnection of the spinal sympathetic neurons from cerebral control represents a unique possibility for analysis of the sympathetic influence on the heart rate (HR) variability (HRV).
In patients with myocardial infarction, diabetic neuropathy, heart failure, and those after cardiac transplantation and SCI, a reduced HRV is consistently observed.4 It is the goal of the present study to evaluate the changes of the ANS by HRV analysis following an acute SCI.
Heart rate variability
HRV can be measured in time and frequency domain analyses. The time domain analysis examines the variability of time segments between heartbeats. It is measured as beat-to-beat intervals or as variables derived from differences in adjacent normal-to-normal sinus (NN) intervals. The parameters include the standard deviation of the average NN intervals (SDANN), the square root of the mean squared differences of successive NN intervals (RMSSD) and the proportions of successive NN interval changes greater than 50 ms divided by the total number of NN intervals (pNN50). SDANN is a very sensitive index of low frequencies and is often used as an overall marker of the ANS activity.5 The RMSSD and pNN50 estimate high frequency variations in the HR.
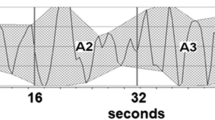
Power spectral analysis (PSA) is measured in the frequency domain. The PSA provides information on the HR distribution as function of frequency and is proposed to be able to discriminate between the sympathetic and parasympathetic contributions of HRV.6 When using a Fast Fourier Transformation algorithm, the RR intervals in the electrocardiogram are transformed into bands with different spectral frequencies (Figure 1). The PSA divides the variability of the HR into Very Low-Frequency Power (0.003–0.04 Hz), Low-Frequency Power (LFP; 0.04–0.15 Hz) and High-Frequency Power (HFP; 0.15–0.4 Hz).6
R-R tachogram and Power Spectral Domain spectrogram by Fast Fourier Transformation. (a) Tachogram displaying a 24-h time series of R-R intervals in a spinal cord injury patient. The tachogram is from a Holter 3 monitoring. (b) Power spectral domain (Frequency domain) analysis of Holter 3 in the same patient by use of Fast Fourier Transformation. The frequency bands at 0.0–0.07, 0.12–0.18 and 0.32–0.34 can, respectively, be interpreted as the Very Low-Frequency Power, the Low Frequency Power and the High Frequency Power. ms, milliseconds; PSD, Power Spectral Density; n.u., normalised units.
The HFP is normally defined as a marker of vagal modulation, that is, determined by the frequency of breathing. LFP is a more controversial component considered to represent the sympathetic nervous system, or a combination of sympathetic and parasympathetic activity.5, 7 The LFP over HFP ratio (LF/HF-ratio) is suggested to reflect the global sympathovagal balance.
Materials and Methods
Rigshospitalet, Copenhagen, Denmark receives all patients with acute traumatic SCI from the Eastern part of Denmark, that is, an up-take area corresponding to a population of 2.5 million—nearly half of the Danish population.
Patient selection
The inclusion started 1st January 2010 and ended 9th March 2013. The inclusion criteria were: (i) traumatic SCI, (ii) neurological level C1-T12, (iii) ⩾18 years and (iv) signed informed consent. The exclusion criteria were: (i) pregnancy, (ii) cardiac pacemaker prior to the traumatic SCI and (iii) concomitant brain injury, that is, Glasgow Coma Score<14. All patients admitted with traumatic SCI were screened for inclusion. The Scientific Ethics Committee of the Capital Region, Denmark, approved the protocol (H-A-2009-065).
Data acquisition
Holter recordings (Lifecard CF digital Holter recorders, Spacelabs Healthcare, Snoqualmie, WA, USA) documented cardiac rhythm continuously for 24 h. The first Holter recording (Holter 1) was obtained as quickly as possible after admission. Holter 2–5 were obtained consecutively as close as possible to 1 week (Holter 2), 2 weeks (Holter 3), 3 weeks (Holter 4) and 4 weeks (Holter 5) after SCI. Arrhythmias from the same recordings have been described.8
HRV analyses
Two experienced readers of eletrocardiograms analysed the Holter recordings using Pathfinder (v. 9.27, Spacelabs Healthcare). Electrocardiogram artefacts like ectopic beats, arrhythmic events, missing data and noise were found by the software and removed after visual verification. The data were exported to Nevrokard Long-Term aHRV analysis software (Nevrokard Kiauta, k.d., Izola, Slovenia). The Nevrokard software removed aberrant beats, HRV-excluded inhibited beats and paced beats to minimise artefacts. An RR-interval tachogram was generated from the continuous electrocardiogram, and a 2048-point Fast Fourier Transformation was performed. Spectral leakage caused by lacking RR intervals at truncated data segments was minimised using a Hanning window tapering function. HRV analyses in the time domain included SDANN, RMSSD and pNN50.
The PSA included Very Low-Frequency Power, LFP and HFP. LF/HF-ratio and Total Power were calculated. The power was normalised to reduce the effects of noise due to artefacts and minimise the effects of the changes in Total Power.5
The time domain and PSA variables were averaged over a 24-h period for the statistical analyses.
Data analysis
A test of normality for all variables was performed graphically using the Kolmogorov–Smirnov test. Variables not normally distributed were logarithmically transformed. Linear mixed model was used to test for significant main effects of the variables over time. Stratified analyses were adjusted for gender, age, previous cardiovascular history, bradycardia, level of injury, ventilator treatment and American Spinal Injury Association Impairment Scale (AIS) grade. The statistical significance of the differences of the mean variables was analysed by one-way analysis of variance with Bonferroni post hoc comparisons. Proportions were compared with χ2-test and Fisher’s exact test. Continuous distributed variables were compared with Student’s t-test. Statistical significance was set at P<0.05. IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA) was used.
Results
Fifty patients with acute traumatic SCI were included, 39 had C1-C8 SCI, 5 had T1-T5 SCI and 6 had T6-T12 SCI.
Four patients experienced one or more episodes of cardiac arrest. Demographic characteristics of the patients are given in Table 1.
Time domain analysis
The time domain analysis showed a rise in SDANN over the first month after SCI (P =0.008), but no variation over time in pNN50 and RMSSD. Figure 2 summarises the mean values of the variables. Stratified analyses by C1-C8, T1-T5 and T6-T12 SCI showed a significant increase in SDANN over time for the C1-C8 (P=0.044) and T1-T5 groups (P=0.036). The analyses were adjusted for age, gender, previous cardiovascular history, ventilator treatment, bradycardia (sinus bradycardia<50) and AIS grade. Similar analyses for the AIS grades showed no rise in AIS A patients (P=0.61), whereas SDANN increased over time in AIS C (P=0.002) and AIS D patients (P=0.007). Adjusted stratified analysis for cardiac arrest events showed a significant rise in SDANN in the group not experiencing cardiac arrest (P<0.005) in contrast to the group experiencing one or more episodes of cardiac arrest (P=0.94).
Mean values in time domain analysis over time. Mean values of time domain variables over time calculated from 24 h Holter recordings as soon as possible after the SCI (Holter 1), after 1 week (Holter 2), 2 weeks (Holter 2), 3 weeks (Holter 4) and 4 weeks (Holter 5) after the SCI. ms, milliseconds; n.u., normalised units. *P<0·05.
When comparing the mean values of the variables, significant differences between the C1-C8, T1-T5 and T6-T12 SCI patients were seen in RMSSD (P=0.003) and pNN50 (P<0.001) (Table 2).
Frequency domain analysis
Mixed model analysis for the first month after SCI showed slight decrease in mean values over time in LFP (P=0.046). HFP, LF/HF-ratio, Very Low-Frequency Power and Total Power showed no significant variation over time. The analyses were adjusted for age, gender, previous cardiovascular history, ventilator treatment, level of injury, bradycardia (SB<50) and AIS grade.
When comparing the mean values of the variables for the five Holter recordings together, several significant differences between the C1-C8, T1-T5 and T6-T12 SCI patients were seen (Table 2).
LF/HF-ratio, Total Power and the LFP showed lower means in most observations in C1-C8 patients compared with the T1-T5 and T6-T12 patients. Higher HFP mean values were seen in C1-C8 SCI patients compared with T1-T6 and T6-T12 SCI patients (Figures 3a–d).
Frequency domain variables over time in relation to level of the SCI. (a) Low Frequency power. (b) High frequency power. (c) Low frequency/High frequency-ratio. (d) Total Power. Mean values of frequency domain variables over time calculated from 24 h Holter recordings as soon as possible after the SCI (Holter 1), after 1 week (Holter 2), 2 weeks (Holter 2), 3 weeks (Holter 4) and 4 weeks (Holter 5) after the SCI in relation to level of the SCI. n.u., normalised units.
Discussion
With this study of HRV, the function of the ANS in the first month after traumatic SCI is assessed. This is the first study to show significant changes in HRV parameters after acute SCI depending on the neurological level and completeness of the SCI.
Time domain analysis
The main finding in the time domain analysis is a significant rise in SDANN during the first month. When stratified by neurological level, the rise is only significant in the C1-C8 and T1-T5 groups. When stratifying by AIS grades, the rise was only seen in incomplete injuries. A review assessing the cardiovascular function in SCI patients concludes that there is no consensus whether cardiovascular function differs between complete and incomplete SCI.9
Our results suggest a rise in the activity in the low frequencies throughout the observation period. The rise in SDANN could be interpreted as a rise in the sympathetic influence on the heart. As patients with acute cervical and high-thoracic SCI experience decreased HRV, the most likely explanation for the rise in SDANN is a recovery of autonomic function towards the habitual autonomic function existing before injury. A significant rise in SDANN is observed in the incomplete C1-T5 patients only. This corresponds with the fact that SCI patients with lesions above C8 often experience a decentralization of the sympathetic nervous system whereas the vagal contribution to the heart remains. As the sympathetic innervation to the heart originates from T1-T5, the significant rise in SDANN in T1-T5 SCI patients might be due to a more direct impact on the cardiac sympathetic preganglionic neurons. The physiological mechanism for the rise of SDANN over time could, in the case of incomplete lesions, be due to spontaneous functional recovery based on synaptic plasticity, remodelling of damaged axons or reactivation of depressed autonomic reflexes, and recovery from the neurogenic shock.10 As expected, T6-T12 patients with intact sympathetic innervation to the heart did not show significant changes in SDANN. Patients with one or more episodes of cardiac arrest did not show a rise in SDANN over time. Either these episodes may be due to a low sympathetic impact on the cardiovascular system or they reflect the underlying cause for the lack of increase in SDANN, which is consistent with findings showing a diminished HRV after acute myocardial infarction.11
No changes over time were seen in pNN50 and RMSSD. Being markers of high-frequency oscillations mainly induced through the intact vagus nerve, no changes were expected.
Frequency domain analysis
Sympathetic influence is found mainly involved in the contribution to LFP. A study by Inoue et al.12 showed a disappearance of LFP in 9 out of 15 tetraplegic SCI individuals. Other studies have likewise demonstrated loss of LFP in SCI patients.2, 13, 14
Pagani et al.19 showed that chronic, but not acute, β-adrenergic receptor blockade reduced LFP at rest; thereby suggesting that sympathetic innervation of the heart is instrumental in the genesis of the LFP component.
Our study shows that low-frequency oscillations are evident in all acute SCI patients including patients with C1-T5 and AIS A injuries where deprivation of sympathetic influence to the heart is expected. This suggests that parasympathetic mechanisms contribute to the mediation of LFP, as a functional reorganisation of spinal cord circuitry affecting sympathetic outflow is more likely to occur in the chronic phase after SCI.10 Other studies agree that LFP partly represents parasympathetic activity, suggesting it to be due to oscillations of the vagal outflow generated by the baroreceptor reflex, driven by sympathetically induced blood pressure low-frequency waves.15
When comparing C1-C8, T1-T5 and T6-T12 patients, we found LFP was lower for C1-T5 lesions in all the recordings. This could be due to interruption of spinal pathways conveying supraspinal signals, and thus emphasise that sympathetic activity, either directly or through alterations in the sympathovagal balance, is important in the interpretation of LFP.
Patients with T1-T5 and T6-T12 SCI have lower HFP values than C1-C8 patients suggesting different mechanisms of vagal outflow depending on whether the injury affects the sympathetic modulation (C1-C8) or the preganglionic sympathetic efferent fibres directly (T1-T5). This difference might explain the unexpected but higher mean values of SDANN in T1-T5 patients were also reported previously.16
All our observations show a lower mean in LF/HF-ratio in the C1-T5 group compared with the T6-T12 group supporting that diminished sympathetic activity in these patients result in lower values of LFP. Likewise, within the C1-T5 group, the mean values in LF/HF-ratio are generally lower in C1-C8 patients than in T1-T5 patients, which could reflect a less affected sympathovagal balance in the latter group. Our results suggest that LFP is partly mediated by sympathetic fibres, which support the assumption that LF/HF-ratio can serve as a measure for the sympathovagal balance as HFP is mediated entirely by vagal control.17, 18, 19
Grimm et al.20 conclude that the two divisions of the ANS can maintain homeostasis in chronic SCI patients even when one component is compromised. We show that this is not applicable in the acute phase after SCI, where the LF/HF-ratio is significantly lower in C1-T5 patients.
Because Total Power reflects all the frequency bands and therefore all the cyclic components responsible for variability, the overall higher mean values in T6-T12 patients were expected (Table 2, Figure 3d). The different outcomes in Total Power and SDANN suggest that SDANN cannot be interpreted merely as an indicator of overall ANS activity but rather should be seen as an index of certain sympathetic components.
Limitations
Although our study includes a larger cohort than most other comparable studies, it is still a limited number of patients, and the comparisons between the C1-C8, T1-T5 and T6-T12 SCI groups may be judged with caution. No AIS B patients were included in the study.
The distribution between AIS groups may vary from study to study, for example, in a study by Scivoletto et al.,21 only 7% of 284 consecutive, newly injured SCI patients were classified as having AIS B. In Denmark, patients having a traumatic SCI may initially be admitted to the nearest hospital, but will afterwards within hours be transferred to one of the two SCI-units covering East or West Denmark. Therefore, the day of admission will nearly always be the day of injury. The exact days after SCI before the patients were equipped with the Holter monitors varied but were accounted for by using days after SCI as the independent variable in Mixed Model Analyses. The use of drugs, that is, sedatives, β-blockers and antiarrhythmic agents, might have an influence on the HRV. This was not analysed because these patients were acutely in need of this treatment and in intensive care units, where the drug administration may often include many and rapid changes making an evaluation questionable.
Overall differences in body position throughout the day or systematic differences in breathing could affect our data. In an effort to overcome this, we adjusted for potential confounders such as use of ventilator treatment in our statistical analyses.
Conclusion
The present study demonstrated a significant rise in SDANN over time for C1-T5 SCI patients. This could, in patients with incomplete lesions, be due to spontaneous functional recovery based on synaptic plasticity or remodelling of damaged axons.
Only patients not suffering from cardiac arrest experienced a significant rise in SDANN over time.
Comparison between C1-C8, T1-T5 and T6-T12 SCI patients suggests that the ANS dysfunction differs between the C1-T5 and T6-T12 patients and even more remarkable between the C1-C8 and T1-T5 patients. Within the first 4 weeks after SCI, a sympathovagal homeostasis has not yet been reached as observed in chronic SCI patients. HRV could potentially be useful to assess recovery of autonomic function in individuals with high thoracic and cervical lesions after acute SCI.
The stratified analyses in this study have limited statistical inference because of the small sample size, and future studies with frequency domain analysis in especially incomplete acute SCI patients are warranted. By comparing larger subgroups of C1-C8, T1-T5 and T6-T12 SCI patients, further changes in the different frequency domain parameters are anticipated and could in the long term be useful as a clinical utility in the diagnosis, prognosis and treatment of SCI individuals.
Data Archiving
There were no data to deposit.
References
Teasell RW, Arnold JM, Krassioukov A, Delaney GA . Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000; 81: 506–516.
Wang YH, Huang TS, Lin JL, Hwang JJ, Chan HL, Lai JS . Decreased autonomic nervous system activity as assessed by heart rate variability in patients with chronic tetraplegia. Arch Phys Med Rehabil 2000; 81: 1181–1184.
West CR, Crawford MA, Poormasjedi-Meibod MS, Currie KD, Fallavollita A, Yuen V et al. Passive hind-limb cycling improves cardiac function and reduces cardiovascular disease risk in experimental spinal cord injury. J Physiol 2014; 592 (Pt 8): 1771–1783.
Okada M, Matsuto T, Satoh S, Igarashi S, Baba M, Sugita O et al. Role of pulse wave velocity for assessing autonomic nervous system activities in reference to heart rate variability. Med Inform (Lond) 1996; 21: 81–90.
Sztajzel J . Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly 2004; 134: 514–522.
Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996; 17: 354–381.
Malliani A, Lombardi F, Pagani M . Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J 1994; 71: 1–2.
Bartholdy K, Biering-Sorensen T, Malmqvist L, Ballegaard M, Krassioukov A, Hansen B et al. Cardiac arrhythmias the first month after acute traumatic spinal cord injury. J Spinal Cord Med 2014; 37: 162–170.
West CR, Bellantoni A, Krassioukov AV . Cardiovascular function in individuals with incomplete spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 2013; 19 (4): 267–278.
Raineteau O, Schwab ME . Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2001; 2: 263–273.
Kuch B, Parvanov T, Hense HW, Axmann J, Bolte HD . Short-period heart rate variability in the general population as compared to patients with acute myocardial infarction from the same source population. Ann Noninvasive Electrocardiol 2004; 9: 113–120.
Inoue K, Ogata H, Hayano J, Miyake S, Kamada T, Kuno M et al. Assessment of autonomic function in traumatic quadriplegic and paraplegic patients by spectral analysis of heart rate variability. J Auton Nerv Syst 1995; 54: 225–234.
Bunten DC, Warner AL, Brunnemann SR, Segal JL . Heart rate variability is altered following spinal cord injury. Clin Auton Res 1998; 8: 329–334.
Claydon VE, Krassioukov AV . Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. Am J Physiol Heart Circ Physiol 2008; 294: H668–H678.
Jokkel G, Bonyhay I, Kollai M . Heart rate variability after complete autonomic blockade in man. J Auton Nerv Syst 1995; 51: 85–89.
Rosado-Rivera D, Radulovic M, Handrakis JP, Cirnigliaro CM, Jensen AM, Kirshblum S et al. Comparison of 24-hour cardiovascular and autonomic function in paraplegia, tetraplegia, and control groups: implications for cardiovascular risk. J Spinal Cord Med 2011; 34: 395–403.
Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ . Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981; 213: 220–222.
Malliani A, Pagani M, Lombardi F, Cerutti S . Cardiovascular neural regulation explored in the frequency domain. Circulation 1991; 84: 482–492.
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986; 59: 178–193.
Grimm DR, De Meersman RE, Almenoff PL, Spungen AM, Bauman WA . Sympathovagal balance of the heart in subjects with spinal cord injury. Am J Physiol 1997; 272 (2 Pt 2): H835–H842.
Scivoletto G, Morganti B, Molinari M . Neurologic recovery of spinal cord injury patients in Italy. Arch Phys Med Rehabil 2004; 85: 485–489.
Acknowledgements
The study has been supported by grants from the Lundbeck Foundation, the Toyota Foundation and the Tryg Foundation, Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Malmqvist, L., Biering-Sørensen, T., Bartholdy, K. et al. Assessment of autonomic function after acute spinal cord injury using heart rate variability analyses. Spinal Cord 53, 54–58 (2015). https://doi.org/10.1038/sc.2014.195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.195
This article is cited by
-
Transcriptomic analysis of α-synuclein knockdown after T3 spinal cord injury in rats
BMC Genomics (2019)
-
Relationship between autonomic cardiovascular control and obstructive sleep apnoea in persons with spinal cord injury: a retrospective study
Spinal Cord Series and Cases (2018)
-
Influence of neurological lesion level on heart rate variability and fatigue in adults with spinal cord injury
Spinal Cord (2016)
-
Cardiovascular autonomic control in paraplegic and quadriplegic
Clinical Autonomic Research (2016)
-
Estimating the autonomic function from heart rate variability in mechanically ventilated patients after spinal cord injury
Spinal Cord (2015)