Abstract
Objectives:
The aim of this study was to determine the therapeutic effects of a collagen scaffold-collagen binding brain-derived neurotrophic factor (CBD-BDNF) complex (CSCB) on behavioral, electrophysiological and histological improvements in canine complete spinal cord transection model.
Methods:
A total of 24 adult female beagle dogs received a complete spinal cord transection at the T12 level in three groups, including SHAM group (n=8), CTL group (complete spinal cord transection without any treatment, n=8) and CSCB group (complete spinal cord transection with CSCB, n=8).
Results:
CSCB therapeutic group showed markedly functional recovery by Olby score and spinal somatosensory evoked responses (SSERs) assay at 12 weeks after complete spinal cord transection. Furthermore, numerous peripheral myelinated axons aligned parallel to the long axis of the spinal cord were detected in CSCB group dogs. In stark contrast, the injured site was dominated by fibrous and only scattered axons were detected in the edge of spinal cord in CTL group dogs.
Conclusion:
The CSCB has an evident therapeutic effect by facilitating peripheral nerve infiltrating following the severe spinal cord injury in canine animals.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI), leading to permanent loss of sensory and motor functions, is a serious disease.1 It is well known that axonal ingrowth in SCI is very limited and developing effective therapeutic treatment is still a major clinical challenge.2 The inhibitory environmental components like cystic cavity formation, glial scar and deficiency of neurotrophic factors in SCI create a physical and molecular barrier to impede axonal ingrowth and exacerbate the neurological deficits.3 The provision of necessary mechanical substrate guidance and appropriate neurotrophic support for axonal ingrowth is essential for facilitating functional recovery in SCI. Thus, our research is focused on developing a combined complex of mechanical substrate guidance scaffold and neurotrophic factors for SCI therapy.
Previously, we had showed linear ordered collagen scaffold fibers (LOCS) provided directional guidance for axonal growth and also acted as a backbone to carry drugs.4 Collagen binding brain-derived neurotrophic factor (CBD-BDNF) is a recombinant fusion protein consisting of active brain-derived neurotrophic factor (BDNF) and a peptide ‘TKKTLRT’ deduced from collagenase as collagen-binding domain (CBD), which could specifically bind the collagen to limit rapid diffusion and had a synergistic therapeutic effect in rat SCI model.5 This collagen scaffold-CBD-BDNF complex (CSCB) had much better recovery, such as reducing cystic cavity, and extending axonal growth, compared with only collagen scaffold treatment.5,6
It is important to test CSCB therapy in large animals or non-human primates SCI before clinical trial.7,8 From the anatomical and physiological perspective, non-human primates are good candidates for SCI model, but high expense and difficult post-surgery care preclude their popularization.9 Therefore, dogs may be a good candidate for the suitable large animal SCI model becasue of big size, easily neurological examinations and easy post-operative care. Thus in this study, we implanted CSCB in the canine complete spinal cord transection model to evaluate its therapeutic effects on axonal ingrowth and functional recovery.
Materials and methods
Ethics statement
Animal experiments were performed in accordance with Guide for the Care and Use of Laboratory Animals from National Institutes of Health and approved by the Animal Care and Use Committee of Nanjing University.
CSCB preparation
As previously described, LOCS fibers were prepared from bovine aponeurosis10 and the diameter of a single LOCS fiber was about 50–200 μm and was made up of a number of finer micro-fibers. LOCS showed good biocompatibility to DRG cells and provided guidance cues, allowing the DRG neurites directional growth on its’ fibers. CBD-BDNF was prepared as described before5 and a bundle of LOCS fibers (about 800–1000) was incubated with 5.6 nmol of CBD-BDNF in 50 μl distilled water 1 h before implantation. The affinity-based binding of CBD-BDNF to LOCS constructed the CSCB (Figure 1).
Characterization of collagen scaffold-CBD-BDNF complex (CSCB). (a) The photo of LOCS fibers. Scale bar=1 cm. (b) s.e.m. image exhibiting microstructure of a single LOCS fiber. Scale bar=30 μm. (c) Fluorescence image of DRG cells growing along the fibers of LOCS. Scale bar=200 μm. (d) The photo of CSCB used for canine SCI model. Scale bar=5 mm.
Complete spinal cord transection
A total of 24 adult female Beagle dogs (Anlimo Biotechnology Inc., Nanjing, China) were received complete spinal cord transection as previous reported.5 After dorsal laminectomy at the T12 vertebral level, a 4–5 mm segment was excised, leaving a gap about 7 mm due to retraction of the nerve ends. A 7 mm-long and 5 mm-diameter bundle of CSCB fibers binding with 5.6 nmol CBD-BDNF was implanted in the gap and aligned with the spinal cord. A collagen membrane was placed over the two stumps to reduce peridural adhesion. According to the implants between the transected sites, all animals were allocated into three groups: SHAM group (n=8), Control (CTL) group without implants (n=8); CSCB group with CSCB implantation (n=8). After surgery, dogs received antibiotics and glucose intravenously for 7 days. Dogs lacked normal micturition reflex and their bladders were manipulated twice a day.
Olby score assessment
Olby score was used to evaluate functional motor recovery.11 During assessment, each dog was walking on the floor for a minimum of 10 steps from both sides and behind. Using the 15-point scoring system, the dogs’ gait was independently scored from two individuals unaware of the experimental conditions. The mean score was calculated from each dog at 4, 8 and 12 weeks after surgery.
Spinal somatosensory evoked responses (SSERs)
Spinal somatosensory evoked responses (SSERs) were performed to check the electrophysiological activity as previous description.12 Briefly, two stimulating electrodes were inserted into the tibial nerve, and two recording electrodes were positioned in the spinal cord at the level of T10 and L1. A reference electrode was inserted subcutaneously, half way between stimulating and recording electrodes. The two recording electrodes recorded the amplitudes of caudal SSERs and rostral SSERs, respectively. Rostral SSERs (mV)/caudal SSERs (mV) represented the electrophysiological recovery degree.
Histological analysis
All dogs were sacrificed at 12 weeks after surgery. The spinal cords from T11–T13 were retrieved and fixed in 4% (v/v) formaldehyde for 48 h. The segments were then embedded in paraffin and cut into 5-μm thick sections by Lecia RM2235 (Leica Biosystems, Wetzlar, Germany). Adjacent tissue sections were stained with hematoxylin and eosin (H&E) for general observation of cellular and extracellular matrix features, Masson’s trichrome, which was used to identify the presence of collagenous tissue within the defect, and the Luxol Fast Blue (LFB) staining, which was used to identify myelin in regenerated nervous tissue.
For immunofluorescence staining, the paraffin embedded sections were subjected to heat mediated antigen retrieval using citric acid, and the sections were then blocked using normal horse serum/T-PBS (1:20) for 15 min. Primary antibodies were applied to the sections and incubated overnight at 4 °C. The primary antibodies were against the following: neurofilament 200 kDa (1:200, ab3966, Abcam, Cambridge, UK), GFAP (1:800, ab7779, Abcam) and 5-HT (1:800, 20079, ImmuoStar, Hudson, WI, USA). NF and GFAP were double labeled on slides. Sections were then incubated with secondary antibody (Alexa Fluor 488, 1:400; Alexa Fluor 594, 1:800; Invitrogen, Carlsbad, CA, USA). Cell nuclei were stained with DAPI (1:1000, Sigma-Aldrich, St Louis, MO, USA) and images were taken under the Leica TCS SP8 Confocal Microscope (Leica Microsystems, Wetzlar, Germany).
Quantitative analyses
Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) was used to quantify neurofilament staining-positive area percentage (positive staining area/total area) by randomly selecting at least 15 fields per sample at × 200 magnification. The re-myelination of the regenerated nerves was observed by LFB staining and transmission electron microscope (TEM). The number of myelinated axons was quantified from at least 10 randomly selected fields per sample under × 1500 magnification. The myelinated axon diameter and thickness of myelin sheath were also evaluated by Image-Pro Plus software (Media Cybernetics).
Statistical analysis
Data were presented as mean values±s.d. SPSS 13.0 (SPSS, Chicago, IL, USA). was used for all statistical analysis. Olby scores for each group were analyzed using repeated measures two-way ANOVA. The LSD test was used for post hoc analysis to correct for multiple comparisons. Other comparisons data were performed with one-way ANOVA (S-NK). P values <0.05 were considered significant.
Results
CSCB enhanced functional recovery after canine SCI
Firstly, we used Olby scoring system to check the functional recovery of hindlimb based on pelvic limb gait of dogs (Figure 2a). All dogs in the CSCB and CTL groups were completely paralyzed (Olby score: 0) immediately after surgery. The recovery in the CTL group was very slow and the score was modestly enhanced from 4–12 weeks after surgery. However, the score in the CSCB group kept increasing and was higher than that in CTL group at indicated time points (P<0.05). These results showed that CSCB had improved functional recovery in the canine SCI model. Consistent with Olby scoring assay, SSERs assay also showed CSCB promoted the recovery of electrophysiological activity. Dogs in CSCB group also had markedly higher SSERs (72.7±7.6%) than that (43.1±3.3%) in CTL group (Figure 2b). These results confirmedly showed CSCB could enhance hindlimb movement recovery as well as electrophysiological recovery.
CSCB reduced the collagen deposition
Masson’s trichrome staining showed that a large quantity of collagen deposition was dominantly filled in the defect at 12 weeks post surgery in the CTL group. In stark contrast, the collagen deposition staining was remarkably less in the CSCB group, only trace staining in the lesion center (Figures 3a–f). The H&E staining analysis showed disordered structure at the injured site in the CTL group, whereas the regeneration tissue in the CSCB group exhibited linear ordered structure, similar to the SHAM group (Figures 3g–i).
Masson’s trichrome and H&E staining at week 12 after SCI. (a–c) Masson’s trichrome staining in the longitudinal sections of the three groups (Scale bar=2 mm). Collagen deposit was stained blue. (d–f) The magnification of the boxed areas (scale bar=100 μm). (g–i) H&E staining of the boxed areas (scale bar=100 μm).
CSCB enhanced axonal ingrowth
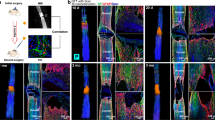
As Figure 4a showed, in the SHAM group, NF positive axons are thin and linear ordered structure, parallel surrounded with astrocytes. While in the groups of CTL and CSCB, astrocytes highly accumulated in rostral and caudal ends, no astrocyte staining in the center of damaged spinal cord. In contrast, there were many NF positive axons in CSCB implant, and the regenerated axons grew along with the direction of CSCB. But there were almost no obviously visible NF positive axons in the injured center and only sporadic axons near the edge of the lesion site in CTL group. The quantified data of NF-staining also showed the NF staining density in CSCB group was significantly higher than that in the CTL group (Figure 4b). Interestingly, we observed with HE staining (Figure 3i) that the organization of the tissue in the CSCB implant resembled peripheral nerve and that more NF positive nerve fibers were present in the CSCB implant than in the SHAM group. These suggested the regenerated nerve fibers possibly grew into CSCB implant from dorsal roots.
Collagen scaffold-CBD-BDNF complex (CSCB) promoted axon ingrowth at week 12 after injury. (a) The overview of immunostaining with neurofilament (NF), Glial fibrillary acidic protein (GFAP) and DAPI specific antibodies in sections of the spinal cord transected sites (scale bar=2 mm). The right panel was the magnification of the boxed areas (scale bar=200 μm). (b) The quantification of NF-positive area of each group (*P<0.05 compared with the SHAM group; ΔP<0.05 compared with CTL group).
It is reported endogenous Schwann cells originating from dorsal and ventral roots could infiltrate into the injury spinal cord.13, 14, 15 To further investigate whether peripheral nerve infiltrating into CSCB implant, we checked Schwann cells infiltrating by S-100 (Schwann cells’ marker) immunochemistry staining. In the center of damaged spinal cord, S-100 staining showed similar pattern with NF-staining and it implied Schwann cells infiltrated into CSCB implant (Figure 5). Because S-100 is also expressed in some astrocytes, many S-100 positive cells could also be seen in the SHAM group.
Nextly, we wanted to check if locomotion nerve also grew in the CSCB implant like the peripheral nerve. 5-HT, produced by neurons in the raphe nuclei of the brain stem, is an important neurotransmitter for voluntary movements.16 Unfortunately, we did not detect 5-HT positive staining in the CSCB implant (Supplementary Figure 1). In short, CSCB implant promoted peripheral nerve ingrowth and Schwann cells infiltrating, which was benefited to the functional recovery of canine SCI accordingly.
CSCB enhanced remyelination
As we know, remyelination of regenerated axons is very important for physiological function recovery.17 Strikingly, the LFB staining showed a lot of myelinated area in the CSCB group, closely similar to the SHAM group. But in the CTL group, the staining signal was very weak, indicating much less myelinated axons, compared with CSCB treatment (Figures 6a–c). To further confirm the LFB staining, TEM analysis was used to check the myelination of regenerated axons (Figures 6d–i). TEM analysis further confirmed that CSCB group had more myelinated axons, bigger diameter of myelinated axons and thicker myelin sheath, compared with the CTL group (Figures 6j–l). Both LFB staining and TEM analysis showed the structure of remyelinated axons were suggestive of peripheral ones. These results indicated CSCB not only enhanced axonal ingrowth, but also promoted myelination of new regenerated axons from a peripheral nerve.
The remyelination of regenerated axons at week 12 after injury. (a–c) Luxol fast blue (LFB) staining in the longitudinal sections (Scale bar=100 μm). (d–f) Transmission electron microscope (TEM) in the ultra-thin sections at low magnification (scale bar=10 μm). (g–i) The magnification of the boxed areas showed detailed remyelination of regenerated axons. (Scale bar=2 μm). ‘*’ indicated the unmyelianted axons. (j–l) The quantification of the myelinated axons number (j), myelinated axon diameter (k) and thickness of myelin sheath (l). (*P<0.05 compared with the SHAM group; ΔP<0.05 compared with the CTL group).
Discussion
Locomotor ability is one of the most urgent needs for the majority of patients with SCI. It is well documented that following SCI, the ingrowth of axon is correlated with the increase in locomotor recovery.18 The Olby score in the CSCB group reached 4.1 after 12 weeks, corresponding to movement of coxae and knee joint of hindlimbs. However, Olby score in the CTL group was only about 2 (less 1-fold than CSCB) until 12 weeks and most of the dogs could not move the joint. For the human afflicted with SCI, such difference could dramatically improve the quality of life. So, CSCB is supposed to potentially improve the functional recovery and improve the life qualify in future clinic application for the SCI patients.
In this study, CSCB markedly inhibited the collagen deposition in the lesion center and significantly enhanced NF-positive nerve fibers regeneration. As an insulator surrounding the axons, the myelin sheath facilitates rapid and efficient transmission of impulses along the nerve cells.17 CSCB also significantly promoted Schwann cells infiltrating and increased the number of myelinated axons, myelin sheath thickness, and the diameter of myelinated fibers in canine SCI. The HE staining (Figure 3i), NF-staining and myelination structure showed the regenerated axons in CSCB implant were from dorsal roots and this might explain the behavioral and sensory function recovery in the CSCB group.
Consistently, CSCB enhanced electrophysiological activity after 12 weeks. Although we failed to detect 5-HT positive nerve fibers in the CSCB implant and regenerated axons did not have obvious connection between the host spinal cord and the tissue in the graft, the CSCB implant still improved the locomotion recovery. Recent publication showed locomotor function recovery could be independent on input from descending motor pathways.19 Previous report also showed the reorganization of segmental, intersegmental, and suprasegmental pathways contribute to recovery of function.14 So, with peripheral nerve infiltrating in CSCB, local circuit without axons and projection neurons maybe form and contribute to locomotion recovery.
Here we showed CSCB implant enhanced axonal ingrowth and functional recovery in most severe SCI model by removing about 5-mm spinal cord. This indicated the new concept like CSCB implant indeed could promote functional recovery in SCI. At 12 weeks post SCI, implanted collagen matrix had mostly degraded according to Masson and HE staining. We also checked immune cells, microglia (Iba1 staining) infiltrating in the CSCB implant; we did not detect obvious deference between CTL group and CSCB group (Supplementary Figure 2). These indicated our collagen scaffold has good biocompatibility, degradability and low immunogenicity. Although CSCB has limitation in human translational approach for the contused or compressed SCI, it is still potentially a useful approach in some cases of severe human SCI through removing necrotic spinal cord and transplanting the CSCB, such as SCI with big defect or large cystic cavity, large mass loss of spinal cord, extreme severe contusion or compression and some chronic injuries.
CSCB provided a good therapeutic effect on canine SCI and we noted that electrophysiological activity was dramatically recovered (SSERs recovery 70% compared with SHAM, Figure 2b) by CSCB, but locomotor movement recovery was very modest (Olby score recovery: only about 30% compared with sham, Figure 2a). This suggests more active rehabilitation therapies such as exercise, special nutrition or careful post-operative care could be very important for enhancing better functional recovery in cases of SCI.
Conclusions
In this study, the CSCB consisting of LOCS fibers and collagen binding neurotrophic protein CBD-BDNF was evaluated in canine completely transected SCI model. The results showed that CSCB significantly improved the behavioral and electrophysiological recovery by facilitating peripheral nerve infiltrating and ingrowth. Therefore, the CSCB was a very promising material for bridging the nerve defect and it may be clinically useful in the future.
DATA ARCHIVING
There were no data to deposit.
References
Harness ET, Yozbatiran N, Cramer SC . Effects of intense exercise in chronic spinal cord injury. Spinal Cord 2008; 46: 733–737.
Giger RJ, Hollis ER 2nd, Tuszynski MH . Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2010; 2: a001867.
Li J, Lepski G . Cell transplantation for spinal cord injury: a systematic review. BioMed Res Int 2013; 2013: 786475.
Cao J, Sun C, Zhao H, Xiao Z, Chen B, Gao J et al. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials 2011; 32: 3939–3948.
Han Q, Sun W, Lin H, Zhao W, Gao Y, Zhao Y et al. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Engi Part A 2009; 15: 2927–2935.
Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J et al. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials 2010; 31: 9212–9220.
Iwanami A, Yamane J, Katoh H, Nakamura M, Momoshima S, Ishii H et al. Establishment of graded spinal cord injury model in a nonhuman primate: the common marmoset. J Neurosci Res 2005; 80: 172–181.
Blesch A, Tuszynski MH . Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci 2009; 32: 41–47.
Jeffery ND, Lakatos A, Franklin RJ . Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma 2005; 22: 1282–1293.
Lin H, Chen B, Wang B, Zhao Y, Sun W, Dai J . Novel nerve guidance material prepared from bovine aponeurosis. J Biomed Mater Res Part A 2006; 79: 591–598.
Olby NJ, De Risio L, Munana KR, Wosar MA, Skeen TM, Sharp NJ et al. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res 2001; 62: 1624–1628.
Saadoun S, Bell BA, Verkman AS, Papadopoulos MC . Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 2008; 131: 1087–1098.
Hejcl A, Sedy J, Kapcalova M, Toro DA, Amemori T, Lesny P et al. HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Devel 2010; 19: 1535–1546.
Woerly S, Doan VD, Evans-Martin F, Paramore CG, Peduzzi JD . Spinal cord reconstruction using NeuroGel implants and functional recovery after chronic injury. J Neurosci Res 2001; 66: 1187–1197.
Woerly S, Doan VD, Sosa N, de Vellis J, Espinosa-Jeffrey A . Prevention of gliotic scar formation by NeuroGel allows partial endogenous repair of transected cat spinal cord. J Neurosci Res 2004; 75: 262–272.
Saruhashi Y, Young W, Perkins R . The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol 1996; 139: 203–213.
Nave KA . Myelination and the trophic support of long axons. Nat Rev Neurosci 2010; 11: 275–283.
Reynolds LF, Bren MC, Wilson BC, Gibson GD, Shoichet MS, Murphy RJ . Transplantation of porous tubes following spinal cord transection improves hindlimb function in the rat. Spinal Cord 2008; 46: 58–64.
van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012; 336: 1182–1185.
Acknowledgements
This work was supported by grants from the ‘Strategic Priority Research Program of the Chinese Academy of Sciences’ (Grant No. XDA01030401), National High Technology Research and Development Program (‘863’ Program) of China (2012AA020501) and National Natural Science Foundation of China (30930032).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Spinal Cord website
Supplementary information
Rights and permissions
About this article
Cite this article
Han, S., Wang, B., Jin, W. et al. The collagen scaffold with collagen binding BDNF enhances functional recovery by facilitating peripheral nerve infiltrating and ingrowth in canine complete spinal cord transection. Spinal Cord 52, 867–873 (2014). https://doi.org/10.1038/sc.2014.173
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.173
This article is cited by
-
Allotransplantation of adult spinal cord tissues after complete transected spinal cord injury: Long-term survival and functional recovery in canines
Science China Life Sciences (2020)
-
Transplantation of adult spinal cord grafts into spinal cord transected rats improves their locomotor function
Science China Life Sciences (2019)
-
Transplantation of adult spinal cord tissue: Transection spinal cord repair and potential clinical translation
Science China Life Sciences (2019)
-
Complete canine spinal cord transection model: a large animal model for the translational research of spinal cord regeneration
Science China Life Sciences (2018)
-
Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury
Scientific Reports (2017)