Abstract
Study design:
Case report.
Objectives:
To report a case with thoracic myelopathy caused by ossification of the posterior longitudinal ligament (OPLL) and ossification of the ligamentum flavum (OLF), in which postoperative paralysis occurred after laminectomy and was reversed after an additional posterior instrumented fusion.
Setting:
A University Hospital in Japan.
Case report:
A 71-year-old woman, with a spastic palsy of both lower extremities, had OPLL and OLF at T10–T11, which pinched the spinal cord anteriorly and posteriorly. She underwent a laminectomy at T10–T11, and no further neurological deterioration was seen immediately after surgery. Over the next 18 h, however, myelopathy worsened, showing severe paraparesis. An additional posterior instrumented fusion at T7–L1 was performed without correction of the kyphosis. After fusion, neurological deficits gradually recovered, despite the presence of residual anterior impingement of spinal cord by the OPLL.
Conclusions:
The present case provides evidence for the possibility that laminectomy alone produces postoperative paralysis for combined thoracic OPLL and OLF, and we recommend that a posterior instrumented fusion should be added when posterior decompression is performed for this disorder.
Similar content being viewed by others
Introduction
Ossification of the posterior longitudinal ligament (OPLL) and ossification of the ligamentum flavum (OLF) have been considered as the same clinical entity as ankylosing spinal hyperostosis and diffuse idiopathic skeletal hyperostosis.1 In the thoracic spine, OPLL sometimes combines with OLF, and severe myelopathy occurs when OPLL and OLF develop at the same vertebral level and pinch the spinal cord anteriorly and posteriorly.
It has been generally accepted that surgery for combined thoracic OPLL and OLF has a high risk for small neurological improvement.2, 3 To date, many surgeons have performed a variety of surgical procedures to treat thoracic myelopathy due to combined OPLL and OLF.3, 4, 5, 6, 7 However, the possibility of postoperative paraplegia remains a risk, and consistent protocols for specific procedures for surgical treatment of combined thoracic OPLL and OLF have not yet been established.
We report a case of thoracic myelopathy due to combined OPLL and OLF, in which paraparesis occurred after laminectomy and was reversed by an additional posterior instrumented fusion. This report provides us with some clues for clarifying the mechanism of postoperative neurological deterioration in cases of combined thoracic OPLL and OLF and develops appropriate surgical protocols for the treatment of this disorder.
Case report
A 71-year-old woman with a 4-year history of gait disturbance was admitted to our institute in August 1998. She could stand up with assistance, but had been unable to walk for 9 months. She had a motor weakness of both legs, showing grade 4/5 muscle strength. She also had a distal sensory loss bilaterally below the inguinal region, showing grade 3–5/10 hypalgesia at thighs, grade 2–3/10 hypalgesia at feet and analgesia at her left toes. Knee and ankle jerks were increased; ankle clonus and bilateral extensor plantar responses were present. She suffered from diabetes mellitus and hypertension, both of which were treated by medication.
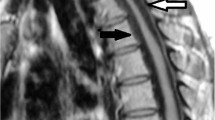
Radiological examination demonstrated thoracic OPLL and OLF at T10–T11. Magnetic resonance (MR) imaging and computed tomography (CT) myelograms revealed that the spinal cord was compressed by OPLL anteriorly (Figure 1, arrowhead) and by OLF posteriorly (Figure 1, arrows) at T10–T11. In the T2-weighted MR image, there was no abnormal intensity in the spinal cord at T10–T11 (Figure 1).
Preoperative radiographic images obtained in a 71-year-old woman with thoracic myelopathy. A midsagittal view of the T2-weighted MR image (left) and axial views of the CT myelogram at T10–T11 (right, upper and lower) demonstrate that the spinal cord compressed by OPLL anteriorly (arrowhead) and by OLF posteriorly (arrows)
The patient was prepared for surgical treatment, which was planned as a laminectomy and extirpation of OLF at T10–T11, with electrophysiologic monitoring of spinal cord activity. At first, we performed a wide laminectomy at T10–T11, which included a resection of the medial two-thirds of the facets. During the procedure, we found severe adhesion of the ossified ligamentum flavum and the dura mater. To extirpate the ossification completely, we had to excise a part of the dura mater. As a result, a defect of the dura mater was formed and cerebral spinal fluid leakage occurred. We repaired the defect with a patch from the fascia of back muscle, and free-fat was grafted over the repaired dura. Intraoperative spinal ultrasonography after the posterior decompression revealed that the spinal cord was compressed anteriorly by the OPLL at T10–T11 (Figure 2, arrow). The spinal cord-evoked potential was stable throughout all the procedures. The incision was closed, and the patient returned to the recovery room.
After surgery, the patient awoke with no neurological deterioration; she showed grade 4/5 muscle strength of both lower extremities. Over the next 18 h, however, her neurological sign rapidly worsened. At 09:00 in the morning following surgery, she reported severe paraparesis, showing grade 0/5 muscle strength. The pinprick test showed analgesia at both lower limbs, but soft touch sensation was slightly preserved at feet. The reflexes of bilateral patellar and Achilles tendons were weak, but detectable. Babinski sign was still positive bilaterally. At 09:30 in the morning, we performed an intravenous bolus administration of 1000 mg methylprednisolone sodium succinate. In addition, we simultaneously started a 2-h intravenous infusion of 1000 mg methylprednisolone sodium succinate. Immediately after the bolus administration, motor function slightly recovered and dorsal and plantar flexion of bilateral toes became possible. At 12:00 (30 min after the administration), however, the effect of the steroid injection vanished, and she could not move her toes at all.
At 13:00 in the afternoon following surgery, the patient was returned to the operating room. The patient underwent additional staged surgery, which was initially planned as an instrumented fusion posteriorly, followed by a corpectomy and an extirpation of OPLL anteriorly through thoracotomy. During the second surgery, we reopened the wound and observed the area of laminectomy at T10–T11. There were no findings of epidural hematoma or tissues that might compress the spinal cord posteriorly. We removed the dura-patch and directly observed the posterior aspect of the spinal cord; however, no abnormal finding was seen at the cord. We reperformed the dura patch and then fused the spine with instrumentation posteriorly from T1–L1 without correction of the kyphosis. The incision was closed, and anterior decompression surgery was planned for 3 weeks later, after evaluating her neurological status and physical condition.
Immediately after the instrumented fusion, no neurological change was seen. After 3 h of the fusion, however, the patient was be able to move her toes. On the morning following the fusion, she could flex her knees. Two weeks postoperatively, she had attained grade 3–4/5 muscle strength at both lower extremities. Since the neurological recovery gradually progressed after the instrumented fusion, we suspended the planned anterior decompression surgery. She could stand up with assistance at 2 months, and walk with aid of a cane at 3 months.
In radiographs after the instrumented fusion, the kyphosis at T7–L1 was 32° (Figure 3), which was the same as that in the preoperative radiographs. MR images 1 week after the fusion demonstrated that the spinal cord was still compressed anteriorly by OPLL at T10–T11. In the T2-weighted images, most areas of the flatted spinal cord at T10–T11 showed a high intensity (Figure 4, arrowhead). In addition, the high-intensity area expanded toward the cranial and caudal direction; there was a high-intensity area at the central portion of the spinal cord at T10 and T11 levels (Figure 4, arrows). The T2-weighted MR images 4 months after the fusion demonstrated that the area showing high intensity was restricted at the central portion of the cord at T10–T11 (Figure 5, arrowhead). The intensity of the spinal cord at T10 and T11 levels returned to normal (Figure 5).
T2-weighted MR images 1 week after the instrumented fusion. A midsagittal view (left) and axial views at T10 (right, upper), T10–T11 (right, middle) and T11 (right, lower) are shown. Most of the areas of the flatted spinal cord at T10–T11 show as high intensity (arrowhead). In addition, the high-intensity area expands toward the cranial and caudal direction; there is a high-intensity area at the central portion of the spinal cord at T10 and T11 levels (arrows)
T2-weighted MR images 4 months after the instrumented fusion. A midsagittal view (left) and axial views at T10 (right, upper), T10–T11 (right, middle) and T11 (right, lower) are shown. The area showing high intensity restricts at the central portion of the spinal cord at T10–T11 (arrowhead). The intensity of the spinal cord at T10 and T11 levels returned to normal
The patient's neurological status was maintained until the final follow-up at 5 years and 9 months; she could walk with a cane, though her gait was spastic. She had grade 4–5/5 muscle strength at both lower limbs. Distal dominant sensory loss remained, showing grade 8/10 hypalgesia at both thighs and grade 2–3/10 hypalgesia at feet.
Discussion
Previous reports show that a one-stage circumspinal decompression anteriorly and posteriorly was effective for thoracic myelopathy due to combined OPLL and OLF.3 However, the surgery itself was considered to be high risk, because of its long operation time and relatively high amount of intra- and postoperative bleeding.4 Tomita et al8 suggested that a two-stage operation should be planned if the risk due to surgery was high. In a recent report, they mentioned that they firstly performed a posterior decompression procedure alone and, about 2 weeks later, they added the anterior decompression procedures depending on the recovery of the patients' physical condition.9
In the present case, the patient was aged and had complications of diabetes mellitus and hypertension, suggesting a relatively high risk for one-stage circumspinal decompression. Fujimura et al5 described a two-stage operation for thoracic OPLL and OLF. In their reports, there were cases in which laminectomy and extirpation of OLF were performed as the first surgery, and anterior decompression through thoracotomy was added on another day. Following Fujimura's suggestion, we initially performed posterior decompression alone for the present case. We planned to add anterior decompression through thoracotomy if her neurological recovery was insufficient.
Unexpectedly, paraparesis progressed at an earlier stage after laminectomy, although progression of thoracic kyphosis was not evident. Postoperative MR images showed that the spinal cord was severely damaged at the site of cord compression by OPLL. This suggests that laminectomy in the present case affected the stability of the thoracic spine, and augmented the damage of the spinal cord that impinged by the OPLL anteriorly.
Previous reports indicate that anterior decompression and interbody fusion (ABF) through thoracotomy did not necessarily produce favorable results when performed as rescue surgery on thoracic OPLL patients whose myelopathy was worsened after laminectomy.2, 7 In some cases, postoperative paraplegia developed after ABF.7 It is quite possible that the severely damaged spinal cord potentially resulting from laminectomy could not further tolerate the anterior procedure of OPLL extirpation.
Taking into account the risks associated with ABF,2, 7 we initially employed a posterior instrumented fusion alone as the rescue surgery for the present case. With this method, we expected to prevent further progression of neurological deterioration by stabilizing the spinal column in the affected area. Although we did not correct the kyphosis, the patient's sensory and motor loss gradually recovered. Thus, we suggest that the instability of the spinal column caused by the laminectomy contributed to the neurological deterioration in the present case. Nevertheless, the patient's neurological recovery steadily progressed after the instrumented fusion and was maintained until the final follow-up at more than 5 years, in spite of the remaining anterior impingement of the cord by the OPLL. Thus, posterior instrumented fusion in the present case was more effective than we expected.
Consequently, the present case provides evidence for the possibility that laminectomy alone produces postoperative paralysis in patients with thoracic myelopathy caused by OPLL and OLF. We recommend that posterior instrumented fusion should be added when posterior decompression is performed for combined thoracic OPLL and OLF.
References
Tsuyama N . Ossification of the posterior longitudinal ligament of the spine. Clin Orthop 1984; 184: 71–84.
Ohtani K, Nakai S, Fujimura Y, Manzoku S, Shibasaki K . Anterior surgical decompression for thoracic myelopathy as a result of ossification of the posterior longitudinal ligament. Clin Orthop 1982; 166: 82–88.
Tomita K, Kawahara N, Baba H, Kikuchi Y, Nishimura H . Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine 1990; 15: 1114–1120.
Abumi K, Kaneda K, Satoh S, Hasegawa K . Choice of surgical procedure for thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. In: Yonenobu K, Sakou T, Ono K (eds). OPLL, Ossification of the Posterior Longitudinal Ligament. Springer-Verlag: Tokyo 1997 pp 175–183.
Fujimura Y, Nishi Y, Nakamura M, Toyama Y, Suzuki N . Long-term follow-up study of anterior decompression and fusion for thoracic myelopathy resulting from ossification of the posterior longitudinal ligament. Spine 1997; 22: 305–311.
Ohtsuka K, Terayama K, Tsuchiya T, Wada K, Furukawa K, Ohkubo M . A surgical procedure of anterior decompression of the thoracic spinal cord through the posterior approach. Seikei Saigai Geka 1983; 8: 1083–1090 (in Japanese).
Yonenobu K et al. Thoracic myelopathy secondary to ossification of the spinal ligament. J Neurosurg 1987; 66: 511–518.
Tomita K, Kawahara N, Takino T . Circumspinal decompression for thoracic ossification of the posterior longitudinal ligament and ossification of the yellow ligament. In: Yonenobu K, Sakou T, Ono K (eds). OPLL, Ossification of the Posterior Longitudinal Ligament. Springer-Verlag: Tokyo 1997 pp 193–199.
Kawahara N et al. Circumspinal decompression for thoracic myelopathy due to ossification of the posterior longitudinal ligament. In: Nakamura K (ed). Investigation Committee Report on the Ossification of the Spinal Ligaments. Japanese Ministry of Health, Labour and Welfare: Tokyo 2003 pp 134–139 (in Japanese).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamazaki, M., Koda, M., Okawa, A. et al. Transient paraparesis after laminectomy for thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. Spinal Cord 44, 130–134 (2006). https://doi.org/10.1038/sj.sc.3101807
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101807
Keywords
This article is cited by
-
Wave changes in intraoperative transcranial motor-evoked potentials during posterior decompression and dekyphotic corrective fusion with instrumentation for thoracic ossification of the posterior longitudinal ligament
European Journal of Orthopaedic Surgery & Traumatology (2019)
-
“U” route transforaminal percutaneous endoscopic thoracic discectomy as a new treatment for thoracic spinal stenosis
International Orthopaedics (2019)
-
Resection of spinous processes can cause spinal cord injury in patient with ossification of the posterior longitudinal ligament in the thoracic spine
Spinal Cord (2014)
-
Transient neurological deficit following midthoracic decompression for severe stenosis: a series of three cases
European Spine Journal (2013)
-
Clinical analysis of thoracic ossified ligamentum flavum without ventral compressive lesion
European Spine Journal (2011)