Abstract
Background:
Despite widespread human exposure to biphenol A (BPA), limited studies exist on the association of BPA with adverse health outcomes in young children. This study aims to investigate the effect of prenatal exposure to BPA on toll-like receptor–induced cytokine responses in neonates and its association with infectious diseases later in life.
Methods:
Cord bloods were collected from 275 full-term neonates. Production of TNF-α, IL-6, and IL-10 were evaluated after stimulating mononuclear cells with toll-like receptor ligands (TLR1-4 and 7–8). Serum BPA concentrations were analyzed by enzyme-linked immunosorbent assay. Bacteria from nasopharyngeal specimens were identified with multiplex PCR and culture method.
Result:
Result showed significant association between cord BPA concentration and TLR3- and TLR4-stimulated TNF-α response (P = 0.001) and that of TLR78-stimulated IL-6 response (P = 0.03). Clinical analysis did not show prenatal BPA exposure to be correlated with infection or bacterial colonization during the first year of life.
Conclusion:
This is the first cohort study that indicated prenatal BPA exposure to play a part in TLR-related innate immune response of neonatal infants. However, despite an altered immune homeostasis, result did not show such exposure to be associated with increased risk of infection during early infancy.
Similar content being viewed by others
Main
Bisphenol A (BPA) are xenoestrogens widely used to manufacture polycarbonate and epoxy resins. These materials are commonly used for lining of beverage or food-storage containers, drinking glasses, water pipes, dental sealants, and medical equipment (1). Although various reports have shown that humans are at regular contact with BPA through air, soil, water, and environmental contact, diet is the primary source of exposure for most adults. However, for infants, additional source of BPA burden might result from in utero exposure. Evidences indicate fetal exposure to BPA to result in modification of immune development later in life. For instance, higher maternal polychlorinated biphenyl levels have been shown to associate with altered immune cell counts or serum immunoglobulin concentrations (2,3). Several researches on experimental animal model have shown BPA to modify the response of several cytokines and chemokine, such as IL-4, IL-5, IL-6, IL-10, IL-13, and TNF-α (4,5,6), suggesting an important immunomodulatory role of BPA in the immune system.
Toll-like receptors (TLRs) are highly conserved components of the innate immune system and play critical roles in early innate responses to various invading pathogens. Beside from important contribution to the first line of defense against pathogens, they also play an important role in directing adaptive immune responses. We had previously shown that neonates are capable of responding adequately to TLR stimulation (7). However, given the fact that the developing immune system in fetus may be highly sensitive to endocrine-disrupting compounds such as BPA, early perturbations may result in altered immune development. Increasing evidences suggest that prenatal window represent a critical period in which the developing immune system may be greatly disturbed. However, despite a handful of studies that suggested early BPA exposure to alter cytokine response in murine model, to our knowledge, there is currently no study that associates prenatal BPA exposure with innate immune function in human newborn. In addition, many experimental studies used high doses and/or routes of exposure that are considered less relevant to actual human exposure. In this study, we aimed to determine whether low and environmentally relevant prenatal BPA exposure is associated with alterations in TLR-induced cytokine responses in neonatal infants. Furthermore, consequences of BPA exposure on susceptibility to infection during early life were also investigated.
Results
Subjects and Demographic Data
Of the 353 enrolled pregnant mothers, 79 neonates were excluded due to premature delivery (n = 45), possible congenital infection (n = 9) and insufficient cord blood volume for laboratory examines, or loss to follow-up (n = 25). A total of 274 participants were eligible for the analysis. Comparing mother–infant pairs included in the study to those excluded, there was no significant characteristic differences between the mothers except that since premature babies were excluded in this analysis, the excluded subjects had lower gestational age and birth body weight, and a higher Cesarean section rate probably due to more complicated obstetrical conditions ( Table 1 ). While these differences were statistically significant, we believe that their effect might not be clinically meaningful, as reports have shown prematurity to influence TLR-induced cytokine response (8,9), thus excluding premature neonates from this study should decrease biased results. Overall, the median BPA concentration was 0.34 ng/ml (25th–75th percentiles: 0.22–0.85 ng/ml) among pregnant mothers and 0.27 ng/ml (25th–75th percentiles: 0.21–0.38 ng/ml) in fetal cord blood. Data from this study were comparable to those reported in the literature (10,11,12). It is worth noting that four pregnant mothers’ working environment was related to manufacturing of plastics and resins; however, compared to other pregnant mothers, their BPA levels (0.11, 0.30, 0.24, and 0.62 ng/ml, respectively) were not elevated.
Association of Cord BPA With Toll-Like Receptor Stimulated Cytokine Response
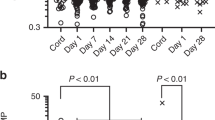
Because the distribution of most cytokine levels and cord BPA concentration were highly skewed (data not shown), we used natural log-transformed cord BPA and TLR-induced cytokine concentration for correlation analysis. The result showed significant association between cord BPA and TLR3 and TLR4-induced TNF-α response (P = 0.001 and P = 0.001) and that of TLR7-8 stimulated IL-6 response (P = 0.03). The result still remained significant after adjusting for potential confounding factors ( Table 2 ). However, none of the TLR-triggered IL-10 productions were associated with cord BPA concentration. Due to the fact that many toxicological studies in animal model suggest a dose-additive effect of chemicals acting upon cytokine response level, we performed a secondary analysis model that divided cord serum BPA concentration into terciles to further explore the interaction between the additive effect of BPA and cytokine response level. The result showed a dose–response relationship between cord BPA and TLR3-triggered TNF-α level, as shown by a trend of decreasing TNF-α response as cord BPA level increased. Similar trend was noted for TLR4-stimulated TNF-α production, revealing significant difference between the first and second tercile of BPA; however, the highest tercile of cord BPA level did not further decrease TNF-α production. Although regression analysis revealed significant association between cord BPA level and TLR7-8-stimulated IL-6 production, the response did not appear to be dose dependent ( Figure 1 ).
Cord mononuclear cells were treated with TLR ligands as described in the Methods. Supernatant was collected for the analysis of cytokines TNF-α and IL-6 production after stimulation with (a) TLR3, (b) TLR4, and (c) TLR7-8 ligands under different BPA level as terciles. The results are expressed by means ± SD. Statistical significance was determined by ANOVA and Dunnett’s post hoc test (*P < 0.05).
Association Between Prenatal BPA Concentration and Nasopharyngeal Bacterial Colonization
Reports have shown that microbial colonization of the airway during early life can modify topical inflammatory mediator release and might have an effect on the risk of infant wheeze (13,14). Since we had observed an association between prenatal exposure to BPA and neonatal immune response, we proceeded to investigate whether the result of altered immune function would lead to modifications in the prevalence of nasopharyngeal bacterial colonization in infants during the first year of life. Assays were performed to identify five different bacterial species from the nasopharynx as described in the Methods. At the age of 1 mo, 42% of the infants were colonized with at least one of the bacteria examined, and methicillin-resistant Staphylococcus aureus accounted for majority of the flora identified (97%). By 12 mo of age, the overall colonization rate was 14.8%, and methicillin-resistant Staphylococcus aureus still remained to be the most common flora identified (54%), followed by Moraxella catarrhalis (16%). Analysis was performed to investigate whether prenatal BPA exposure was associated with nasophyngeal bacterial colonization at age of 1 mo and also by 12 mo. The result, as shown in Table 3 , indicated that cord BPA level had relatively little effect on the prevalence of bacterial colonization in infancy.
Association Between Prenatal BPA Concentration and the Risk of Infection During Early Life
By the age of 1 y, 250 infants had their medical records reviewed and completed the questionnaires administered at 6 and 12 mo of age. Twenty-one participants had lost follow-up, had yet to return, or did not have complete outcome data at the time of analysis. Even though prenatal BPA exposure was not associated with increased bacterial colonization in young infants, we hypothesized that the consequence of BPA-induced cytokine suppression might lead to decreased inflammatory response against microbial invasion, thus increasing the risk of infection. However, statistical analysis did not show significant correlation between cord BPA level and the incidence of infectious diseases such as acute bronchiolitis, pneumonia, acute otitis media, infectious enteritis, and urinary tract infection during the first year of life ( Table 4 ).
Discussion
Results from recent studies have suggested the potential role of endocrine-disrupting chemicals such as BPA to modify immune cytokine response; however, most are results from animal models or in vitro studies that load BPA with dosages higher than usual environmental exposure (15,16,17,18). To our knowledge, this is the first study in humans that investigated the effect of prenatal BPA exposure on neonatal innate immunity at low and environmentally relevant BPA concentration. Our result demonstrated prenatal BPA exposure, one of the most common endocrine-disrupting chemicals, to have a suppressive effect on TLR-induced proinflammatory cytokine response (TNF-α and IL-6) in the neonatal mononuclear cells. TNF-α and IL-6 are pleiotropic cytokines that have substantial importance in inflammatory reaction against microbial invasion. In spite of extensive reports on the immunomodulatory role of BPA exposure, conflicting results exist as some studies showed BPA to prompt inflammatory cytokine production, while others revealed a potential capacity of BPA to suppress cytokine response. One possible explanation for these differing observations is the BPA concentrations used to treat the cells for cytokine production in different study settings. Chao et al. (19) had shown that TNF-α release was inhibited at very high or very low levels of endocrine-disrupting chemical, but the trend of TNF-α is increased at intermediate concentrations. Furthermore, different experimental methodology might also account for the contrasting results. Studies that infer a stimulatory BPA activity with enhanced cytokine production were from a variety of experimentations that treated cells directly with BPA (6,20,21). Results from Zhu et al. and Liu et al. indicated that the addition of BPA in macrophages significantly increased the production of TNF-α and IL-6 through NF-κB signaling pathways (20,21). In contrast, studies that implied inhibitory effect of BPA on cytokine production had experimental settings that pretreated immune cells with BPA followed by infection (bacteria or virus) or stimulation with lipopolysaccharide. The reduction in cytokine production could be explained by reduced neutrophils activity or downregulation of nitric oxide production caused by BPA exposure, thus leading to inappropriate immune response to defend against invading pathogens (15,22,23). In agreement with the later results, we had shown prenatal BPA exposure to reduce TNF-α response to TLR3 and 4 stimulation, and IL-6 response to TLR7-8 in the neonatal mononuclear cell. At present, we do not know whether the influence of BPA on TLR-triggered cytokine response is a result of direct effect of BPA on TLR expression or the result of an indirect effect of BPA that disrupted downstream signaling events. Nevertheless, several experimental studies have shown that BPA can alter cytokine production via the P13K/Akt, the MAPK/AP-1, or the NF-κB pathways, whereas no influence was observed on TLR4 surface expression (21,24,25). The observation from our study that IL-10 response to TLR ligands remained unperturbed by BPA concentration might also give indirect evidence that BPA had no direct effect on TLR expression, because theoretically, all cytokine production would have been altered if TLR expression, being the origin of the signaling pathway, was modified. Further research is needed to explore the precise mechanism of the effect BPA on TLR expression.
Infectious diseases remain among the major causes of illness and hospitalization in young children worldwide. Despite a wide variety of epidemiological and animal studies on the association of BPA with alteration of immune functions and multiple adverse health outcomes (23,26,27), very few researches investigated the effect of BPA exposure on pediatric infectious diseases. While the result from our study did not show prenatal BPA exposure to increase the risk of infection or nasopharyngeal bacterial colonization rate during the first year of life, our finding may contribute to a better understanding of the role of prenatal BPA exposure in childhood infection. Similar to our findings, although Roy et al. (22) found maternal exposure to BPA to alter innate immune response in adult offspring, the modification did not compromise the host’s ability to successfully clear influenza virus from the infected lung. The detailed mechanism underlying these null observations is yet not fully understood but may be explained by several assumptions. First, the fact that since immune system is composed of multiple cells and variable pathways, suppression of certain cytokines of the innate immune system may not have an overall effect in disease outcome. Second, several reports have shown cytokine production to respond to BPA in a dose-dependent manner (15,20,21,23). In some studies in which lower doses of BPA were used, no effect was seen on ex vivo immune cell function or disease progression in vivo (22,28,29). Thus, it is possible that the extent of cytokine suppression exposed to low and environmentally relevant BPA level, such as in our study, was not sufficient to significantly increase the risk of infection or nasopharyngeal bacterial colonization in infants. Third, the few available data that suggest certain pollutants to increase susceptibility to infection were mostly results from animal studies or exposure to environmental toxin other than BPA (such as dioxins, cigarette smoke, diesel exhaust, and other air pollutants) (30,31). To our knowledge, only two published studies implied BPA exposure to increase the risk of infectious diseases in the pediatric population. One study had suggested prenatal BPA exposure to increase the risk of respiratory tract infection in children; however, the association only became significant after adjusting for confounding factors (32,33). Furthermore, whether the effect of BPA on increased airway infection had resulted from direct immunosuppression or an indirect influence inflicted by hyperactive airway remains debatable, since they had concurrent results showing a strong association between BPA exposure and wheeze/asthma. Thus, although BPA can alter immune function, its role in human infectious disease, especially in young children, remains an important issue to be further investigated.
This study has some limitations. First, clinical assessment of infectious disease beyond 12 mo of age was not done. By doing so, and in the context of longitudinal model, would have increased power to detect associations, as the cumulative incidence of infectious disease might increase with growing age. Moreover, this study estimated BPA exposure from serum samples. It has been argued that BPA is rapidly cleared from the blood, thus any data obtained from serum samples may be imprecise or results from contamination (34). Although urine sampling may be less subject to such effects, we did not have access to urine samples from the neonates. However, from the reviews of Vandenberg et al. (35), the 17 studies that measured BPA in blood and serum samples of healthy subjects, most of them had concentrations comparable to the studies of urine. Thus, we proceeded with this work using serum measurements of BPA, and with quality control measures to avoid any contamination during collection and storage. In addition, the use of enzyme-linked immunosorbent assay to measure BPA concentration has been challenged since this method was considered less specific and sensitive than chromatography-related analytical techniques. However, studies have proved that BPA measurements obtained by enzyme-linked immunosorbent assay were within a comparable dose range as the methods that are considered more accurate (36,37); and most importantly, it is much more affordable, simpler, and have a higher throughput. Considering the relatively large quantity of our samples, we had adopted this method, and the results of our BPA measurements were similar to those reported in the literature with either technique. Finally, although cytokine response to TLR ligands have proven to be an important indicator of innate immunity, other measures of immune function, such as immunoglobulin level and cellular immunity, were not included in this study; thus, the results here could not provide a broad view of the consequences of BPA exposure on the entire immune system of the neonatal infants.
In conclusion, this is the first cohort study that indicated prenatal BPA exposure to play a part in the TLR-related innate immune response of the neonatal infant. Although current study did not show developmental exposure to BPA to have a significant impact on the magnitude or functional capacity of the young infant to defend against infection, the significant immunomodulatory effect of BPA in the neonatal immune system should require additional investigations to elucidate its potential effect on the health outcome of young children and its association with immune-related disorders other than infection.
Methods
Study Population
Data for this analysis came from an ongoing prospective birth cohort study called the PATCH (The Prediction of Allergy in Taiwanese Children). The PATCH is an unselected, population-based study designed to investigate the risk factors for developing immune-related and/or allergic diseases in children located in Northern Taiwan. The study was approved by the Chang Gung Ethics Committee, and informed consent was obtained from the parents/legal guardians of the neonates. Pregnant women were approached randomly by a study nurse during their third-trimester clinical visits and invited to join our research program. This analysis comprises the first 353 mother–newborn pairs recruited into the study. Neonates were excluded if born under the gestational age of 37 wk, suspicious of congenital infections (such as maternal or neonatal fever, chorioamnionitis, or respiratory distress), and had major congenital anomaly. A baseline questionnaire survey was conducted at the time of enrollment to obtain parental information such as demographic characteristics, medical and obstetric history, smoking exposure history, and alcohol consumption. Perinatal data such as birth weight and height, infant gender, modes of delivery, and perinatal complications, or infections were obtained from perinatal records. Successive follow-up questionnaires were administered at 6 and 12 mo of age to obtain relevant information such as height and weight measurements, vaccination history, dietary habits, and parental smoking history. Medical history of the children was specifically reviewed with regard to acute bronchiolitis, pneumonia, croup, acute otitis media, infectious enteritis, and urinary tract infection. Infants were defined as ever having an infection if there was a diagnosis from a doctor, and the infant has either been hospitalized or received medical treatment. By the time of analysis, 250 children included in this study were at least 1 y of age and had adequate follow-up data.
Sample Collection and Cell Culture
Umbilical cord blood was obtained at the time of delivery, and mononuclear cells were isolated from heparinized blood by Ficoll-Hypaque (Pharmacia Biotech, Piscataway, NJ) centrifugation. RPMI 1640 supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT), 2 mmol/l glutamine, 100 U/ml penicilline, and 100 μg/ml of streptomycin (complete media) was used for culture (Sigma-Aldrich, St. Louis, MO).
Toll-Like Receptor Ligands Stimulation
TLR ligands used for cell stimulation were obtained from InvivoGen (San Diego, CA) which included synthetic bacterial lipoprotein (PAM3csk4) that is recognized by TLR1-2; a synthetic analog of double-stranded RNA for TLR3 (poly I:C); ultrapure lipopolysaccharide for TLR4; and R848 which activates via the TLR7/TLR8 signaling pathway. Medium without any added ligand was used to determine any baseline production of TNF-α, IL-6, and IL-10. Each serial dilution was performed in duplicate. As a positive control, cells were treated with the NF-kB activator phytohemagglutinin (Murex Pharmaceuticals) at 4 µg/ml in R10-FBS.
To determine TLR responses, 3 × 105 peripheral blood mononuclear cells in 100 µl R10-FBS (or R10-HS, or R10 without serum, where specified) were added to each of the duplicate ligand- or medium-containing wells and incubated at 37 °C for 20 h with 5% CO2. The concentration of 3 × 105 cells/well corresponded to the linear phase of the TNF production curve and was ultimately chosen for subsequent experiments. All assay preparations were performed using sterile technique in a laminar flow hood.
The concentrations of the ligands used for this experiment are as follows: 10 µg/ml of PAM3csk4, 10 µg/ml of poly(I:C), 20 ng/ml of lipopolysaccharide, and 10 µg/ml of R848.
Measurement of Cytokines
TNF-α, IL-10, and IL-6 levels in culture supernatants were determined by enzyme-linked immunosorbent assays (ELISA; R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The detection limits were 15.6 pg/ml for TNF-α, 3.12 pg/ml for IL-6, and 7.8 pg/ml for IL-10.
BPA Concentration
To test for possible contamination of BPA during sample collection and storage, quality control experiments were first conducted. We had initially pooled clean distilled water into empty blood-collecting tubes, stored in BPA-free polystyrene tubes, at −80 °C for many times and performed laboratory tests with exact processing that real samples received. Hence, blood samples were collected and processed according to standard procedures. Detection of total BPA concentration from maternal and cord serum was performed by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (IBL, Gunma, Japan). The detection limit was 1.32 ng/ml.
Bacterial Identification From the Nasopharyx
At the age of 1 and 12 mo, nasopharyngeal specimens were obtained through the nose with separate cotton-tipped swabs (Copan Swab Applicator, Copan Diagnostics, Brescia, Italy). Samples were then transported to the microbiology laboratories within 2 h after collection and cultured for bacteria with the use of standard methods for identification (38). Besides the traditional culture methods, multiplex PCR was also performed as described by Hendolin et al. (39) for simultaneous detection of Streptococcus pneumoniae, Haemophilus influenzae, M. catarrhalis, Streptococcus pyogenes, and S. aureus. For the identification of methicillin-resistant Staphylococcus aureus, Staphylococcus aureus was first identified by coagulase test, and cefoxitin test was conducted subsequently to distinguish methicillin-resistant Staphylococcus aureus from methicillin-susceptible Staphylococcus aureus in accordance with the recommendation of Clinical and Laboratory Standards Institute document M100-S17 (40).
Statistical Methods
Multiple regression analysis was used to determine the relation between neonatal BPA concentration and TLR-induced cytokine response. Neonatal characteristics (birth body height and weight, gestational age, mode of delivery, and gender) and maternal history of allergy, smoking during pregnancy, and maternal education were included in the multiple regression analysis to compensate confounders’ effects. Since the concentrations of cord BPA and cytokines were not normally distributed, values were characterized as terciles or logarithmically transformed as continuous variables in the statistical models. Association between cord BPA concentration and binary outcomes (acute bronchiolitis, pneumonia, croup, otitis media, or infectious enteritis) and nasopharyngeal bacterial colonization were analyzed by using logistic regression. All statistical analysis was carried out using IBM SPSS Statistics Version 20 (Armonk, NY).
Statement of Financial Support
This work was financially supported by Chang-Gung Memorial Hospital, Keelung, Taiwan (grant CMRPG 2B0021-3).
Disclosure
The authors declare no conflict of interest.
References
Teeguarden JG, Hanson-Drury S. A systematic review of bisphenol A “low dose” studies in the context of human exposure: a case for establishing standards for reporting “low-dose” effects of chemicals. Food Chem Toxicol 2013;63:938–48.
Weisglas-Kuperus N, Patandin S, Berbers GA, et al. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ Health Perspect 2000;108:1203–7.
Jusko TA, De Roos AJ, Schwartz SM, et al. Maternal and early postnatal polychlorinated biphenyl exposure in relation to total serum immunoglobulin concentrations in 6-month-old infants. J Immunotoxicol 2011;8:95–100.
Lee MH, Chung SW, Kang BY, et al. Enhanced interleukin-4 production in CD4+ T cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: involvement of nuclear factor-AT and Ca2+. Immunology 2003;109:76–86.
O’Brien E, Dolinoy DC, Mancuso P. Perinatal bisphenol A exposures increase production of pro-inflammatory mediators in bone marrow-derived mast cells of adult mice. J Immunotoxicol 2014;11:205–12.
Holladay SD, Xiao S, Diao H, et al. Perinatal bisphenol A exposure in C57B6/129svj male mice: potential altered cytokine/chemokine production in adulthood. Int J Environ Res Public Health 2010;7:2845–52.
Liao SL, Yeh KW, Lai SH, Lee WI, Huang JL. Maturation of Toll-like receptor 1-4 responsiveness during early life. Early Hum Dev 2013;89:473–8.
Shen CM, Lin SC, Niu DM, Kou YR. Development of monocyte Toll-like receptor 2 and Toll-like receptor 4 in preterm newborns during the first few months of life. Pediatr Res 2013;73:685–91.
Förster-Waldl E, Sadeghi K, Tamandl D, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res 2005;58:121–4.
Kosarac I, Kubwabo C, Lalonde K, Foster W. A novel method for the quantitative determination of free and conjugated bisphenol A in human maternal and umbilical cord blood serum using a two-step solid phase extraction and gas chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2012;898:90–4.
Kuroda N, Kinoshita Y, Sun Y, et al. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J Pharm Biomed Anal 2003;30:1743–9.
Aris A. Estimation of bisphenol A (BPA) concentrations in pregnant women, fetuses and nonpregnant women in Eastern Townships of Canada. Reprod Toxicol 2014;45:8–13.
Tsai MH, Huang SH, Chen CL, et al. Pathogenic bacterial nasopharyngeal colonization and its impact on respiratory diseases in the first year of life: the PATCH Birth Cohort Study. Pediatr Infect Dis J 2015;34:652–8.
Følsgaard NV, Schjørring S, Chawes BL, et al. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med 2013;187:589–95.
Byun JA, Heo Y, Kim YO, Pyo MY. Bisphenol A-induced downregulation of murine macrophage activities in vitro and ex vivo. Environ Toxicol Pharmacol 2005;19:19–24.
Tian X, Takamoto M, Sugane K. Bisphenol A promotes IL-4 production by Th2 cells. Int Arch Allergy Immunol 2003;132:240–7.
Guo H, Liu T, Uemura Y, et al. Bisphenol A in combination with TNF-alpha selectively induces Th2 cell-promoting dendritic cells in vitro with an estrogen-like activity. Cell Mol Immunol 2010;7:227–34.
Yan H, Takamoto M, Sugane K. Exposure to bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect 2008;116:514–9.
Chao TC, Van Alten PJ, Greager JA, Walter RJ. Steroid sex hormones regulate the release of tumor necrosis factor by macrophages. Cell Immunol 1995;160:43–9.
Liu Y, Mei C, Liu H, et al. Modulation of cytokine expression in human macrophages by endocrine-disrupting chemical bisphenol-A. Biochem Biophys Res Commun 2014;451:592–8.
Zhu J, Jiang L, Liu Y, et al. MAPK and NF-κB pathways are involved in bisphenol A-induced TNF-α and IL-6 production in BV2 microglial cells. Inflammation 2015;38:637–48.
Roy A, Bauer SM, Lawrence BP. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS One 2012;7:e38448.
Sugita-Konishi Y, Shimura S, Nishikawa T, Sunaga F, Naito H, Suzuki Y. Effect of Bisphenol A on non-specific immunodefenses against non-pathogenic Escherichia coli. Toxicol Lett 2003;136:217–27.
Calippe B, Douin-Echinard V, Delpy L, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol 2010;185:1169–76.
Lee J, Lee SJ, Lim KT. CTB glycoprotein (75kDa) inhibits IgE releasing, TNF-α and IL-6 expressed by bisphenol A in vivo and in vitro. Food Chem Toxicol 2012;50:2109–17.
Braun JM, Hauser R. Bisphenol A and children’s health. Curr Opin Pediatr 2011;23:233–9.
Rogers JA, Metz L, Yong VW. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol 2013;53:421–30.
Goto M, Takano-Ishikawa Y, Ono H, Yoshida M, Yamaki K, Shinmoto H. Orally administered bisphenol A disturbed antigen specific immunoresponses in the naïve condition. Biosci Biotechnol Biochem 2007;71:2136–43.
Sawai C, Anderson K, Walser-Kuntz D. Effect of bisphenol A on murine immune function: modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ Health Perspect 2003;111:1883–7.
Lawrence BP. Environmental toxins as modulators of antiviral immune responses. Viral Immunol 2007;20:231–42.
Ménard S, Guzylack-Piriou L, Lencina C, et al. Perinatal exposure to a low dose of bisphenol A impaired systemic cellular immune response and predisposes young rats to intestinal parasitic infection. PLoS One 2014;9:e112752.
Gascon M, Casas M, Morales E, et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol 2015;135:370–8.
Kishi R, Kobayashi S, Ikeno T, et al.; Members of the Hokkaido Study on Environment and Children’s Health. Ten years of progress in the Hokkaido birth cohort study on environment and children’s health: cohort profile–updated 2013. Environ Health Prev Med 2013;18:429–50.
Teeguarden J, Hanson-Drury S, Fisher JW, Doerge DR. Are typical human serum BPA concentrations measurable and sufficient to be estrogenic in the general population? Food Chem Toxicol 2013;62:949–63.
Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet 2012;17:407–34.
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–77.
Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 2002;17:2839–41.
Balows A. Manual of Clinical Microbiology. 5th edn. Washington, DC: American society for microbiology, 1991.
Hendolin PH, Paulin L, Ylikoski J. Clinically applicable multiplex PCR for four middle ear pathogens. J Clin Microbiol 2000;38:125–32.
Schwalbe R, Steele-Moore L, Goodwin AC. Antimicrobial Susceptibility Testing Protocols. Florida: Taylor & Francis Group; CRC Press, 2007.
Acknowledgements
The authors thank all the families for their participation.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Liao, SL., Tsai, MH., Lai, SH. et al. Prenatal exposure to bisphenol-A is associated with Toll-like receptor–induced cytokine suppression in neonates. Pediatr Res 79, 438–444 (2016). https://doi.org/10.1038/pr.2015.234
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.234
This article is cited by
-
Sex-dependent dysregulation of human neutrophil responses by bisphenol A
Environmental Health (2021)
-
Effect of bisphenol A on human neutrophils immunophenotype
Scientific Reports (2020)
-
Abnormal differentiation of regulatory T cells and Th17 cells induced by perinatal bisphenol A exposure in female offspring mice
Molecular & Cellular Toxicology (2020)
-
Infant anemia is associated with reduced TLR-stimulated cytokine responses and increased nasopharyngeal colonization with Moxarella catarrhalis
Scientific Reports (2018)
-
Environmental Mechanisms of Neurodevelopmental Toxicity
Current Environmental Health Reports (2018)