Abstract
Background:
Suppressor of cytokine signaling-1 and -3 (SOCS-1 and SOCS-3) are two important negative regulators in the insulin-signaling pathway, and their overexpression may aggravate insulin resistance. Subjects with insulin resistance are often obese and have increased expressions of SOCS-1 and SOCS-3. We speculated that SOCS-1 and SOCS-3 may be involved in abnormal deposition of adipose tissues during insulin resistance.
Methods:
A catch-up growth intrauterine growth retardation (CG-IUGR) rat model with insulin resistance was established; mRNA and protein expression of SOCS-1, SOCS-3, the CCAAT/enhancer binding protein (C/EBPα), and peroxisome proliferator-activated receptor (PPARγ) in adipose tissue were measured by real-time PCR and western blot; plasmids carrying small hairpin RNAs (shRNAs) targeting the SOCS-1 and SOCS-3 genes were constructed and transfected into preadipocytes, which were then induced to mature. At 72 h after differentiation was induced, the expressions of C/EBPα and PPARγ, two important molecules promoting the differentiation of preadipocytes, were detected.
Results:
Expressions of SOCS-1, SOCS-3, C/EBPα, and PPARγ were markedly increased in adipose tissues of CG-IUGR rats, whereas the expressions of C/EBPα and PPARγ were significantly reduced after gene silencing of SOCS-1 or SOCS-3 in adipocytes.
Conclusion:
Overexpression of SOCS-1 and SOCS-3 may enhance the expression of C/EBPα and PPARγ, resulting in abnormal deposition of adipose tissues during insulin resistance.
Similar content being viewed by others
Main
Numerous epidemiological studies have shown that adults with intrauterine growth retardation (IUGR) have a significantly increased risk for obesity and insulin resistance (1,2,3). Our previous studies showed that catch-up growth IUGR rats at the age of 12 wk had developed insulin resistance and overexpressed suppressor of cytokine signaling-1 and -3 (SOCS-1 and SOCS-3); silencing of the SOCS-1 or SOCS-3 gene improved glucose transport in muscle cells (4). Therefore, SOCS-1 and SOCS-3 may be involved in the development of insulin resistance in the muscle cells of IUGR rats. Available evidence suggests that SOCS-1 and SOCS-3 bind to the insulin receptor, inhibiting its phosphorylation and subsequent insulin signal transduction (5,6). In addition, SOCS-1 and SOCS-3 can bind to substrates of insulin receptor-1 and insulin receptor-2, resulting in ubiquitination of insulin receptor substrates, which also inhibits insulin signal transduction (7).
Insulin resistance refers to reduced sensitivity of peripheral tissues to insulin, including muscular tissues and adipose tissues, which have the highest sensitivity to insulin. Adipocytes, the main component of white adipose tissues, not only store energy but are also important endocrine organs involved in the regulation of glucose and fat metabolism (8). Mature adipocytes are differentiated from preadipocytes, which are regulated by transcriptional factors such as the CCAAT/enhancer binding protein (C/EBPα), peroxisome proliferator-activated receptor (PPARγ), and adipocyte determination- and differentiation-dependent factor 1 (ADD1/SREBP1c). PPARγ can induce mRNA transcription of C/EBPα, which may promote PPARγ expression via positive feedback. Furthermore, PPARγ and C/EBPα are essential for the maintenance of differentiation and maturation of adipocytes (9,10). Normal differentiation of adipocytes maintains the balance of energy metabolism, whereas abnormal differentiation of adipocytes may interrupt the effects of insulin, resulting in insulin resistance.
We have successfully established a catch-up IUGR rat model with insulin resistance and constructed plasmids containing shRNA targeting SOCS-1 and SOCS-3. In the current study, we investigated the basal protein levels of SOCS-1, SOCS-3, C/EBPα, and PPARγ in adipose tissues of catch-up IUGR and appropriate-for-gestational-age (AGA) rats. We speculated that SOCS-1 and SOCS-3 may regulate the differentiation of preadipocytes via C/EBPα and PPARγ, leading to the abnormal accumulation of adipose tissues, which is the main cause of insulin resistance in adipose tissues and even the body as a whole.
Results
Increased Expression of Basal SOCS-1 and SOCS-3 Protein in Adipose Tissue of IUGR Rats
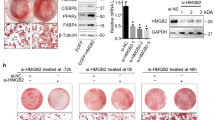
To investigate whether there is a higher SOCS-1 and SOCS-3 protein level in the adipose tissue of IUGR rats at age 12 wk, we examined the subcutaneous and perirenal adipose tissues by western blotting before SOCS-1 and SOCS-3 gene silencing. As compared with the AGA group, the expression of SOCS-1 and SOCS-3 in the subcutaneous and perirenal fat of IUGR rats was dramatically increased (P < 0.05) ( Figure 1 ).
Increased expression of basal SOCS-1 and SOCS-3 protein in adipose tissue of IUGR rats. (a) Crude adipose extracts (50 μg/lane) were analyzed by western blots developed with antibodies to SOCS-1 and SOCS-3; GAPDH blot was included as a loading control. Each lane was from an individual rat. (b) Blots as shown in (a) were quantitated densitometrically and for quantitation of SOCS-1 and SOCS-3. The black bar refers to the IUGR group, whereas the white bar indicates the AGA group. Data are presented as mean ± SD of the relative immunoreactivities (n = 6). Groups indicated as AGA and IUGR. **P < 0.01 as compared with AGA group. AGA, appropriate for gestational age; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IUGR, intrauterine growth retardation; SOCS, suppressor of cytokine signaling.
Increased Expression of Basal C/EBPα and PPARγ Protein in Adipose Tissue of IUGR Rats
C/EBPα and PPARγ basal protein level in adipose tissue was also detected by western blotting in IUGR and AGA rats before SOCS-1 and SOCS-3 gene silencing. As shown in Figure 2 , similar to SOCS-1 and SOCS-3, the protein level of C/EBPα and PPARγ in the subcutaneous and perirenal fat of IUGR rats was markedly increased as compared with the levels in AGA rats (P < 0.05).
Increased expression of basal C/EBPα and PPARγ protein in adipose tissue of IUGR rats. (a) Crude adipose extracts (50 μg/lane) were analyzed by western blots developed with antibodies to C/EBPα and PPARγ; GAPDH blot was included as a loading control. Each lane was from an individual rat. (b) The blots as shown in (a) were quantitated densitometrically and for quantitation of C/EBPα and PPARγ. The black bar refers to the IUGR group, whereas the white bar indicates the AGA group. Data are presented as mean ± SD of the relative immunoreactivities (n = 6). Groups are indicated as control AGA and IUGR. *P < 0.05 as compared with AGA group. AGA, appropriate for gestational age; EBP, enhancer binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IUGR, intrauterine growth retardation; PPAR, peroxisome proliferator-activated receptor.
Downregulation of SOCS-1 Expression Reduces the C/EBPα and PPARγ mRNA and Protein Level in Adipose Tissue
To investigate the relationship between SOCS-1 and the C/EBPα, the expression of PPARγ in adipose tissue, we knocked out the SOCS-1 gene in adipocytes with shRNA-SOCS-1 and then induced it in mature cells. We tested the mRNA expression of C/EBPα and PPARγ by real-time PCR and the protein level by western blotting 3 d after differentiation induction. As shown in Table 1 , the mRNA expressions of C/EBPα and PPARγ were significantly decreased after silencing of the SOCS-1 gene as compared with those of the control group (1.39 ± 0.35 vs. 3.01 ± 0.72, P < 0.05; 1.30 ± 0.36 vs. 2.34 ± 0.18, P < 0.05). C/EBPα and PPARγ protein levels were also markedly reduced after silencing of the SOCS-1 gene as compared with those of the AGA group (P < 0.05) ( Figure 3 ).
Downregulation of SOCS-1 expression reduces the C/EBPα and PPARγ mRNA and protein levels in adipose tissue in the IUGR rats at 3 d after differentiation. (a) Crude adipocyte extracts (50 μg/lane) were analyzed by western blots developed with antibodies to C/EBPα and PPARγ; GAPDH blot was included as a loading control. (b) Blots as shown in (a) were quantitated densitometrically and for quantitation of C/EBPα and PPARγ. The black bar refers to treatment with SOCS-1 shRNA, whereas the white bar indicates negative control. Data are presented as mean ± SD of the relative immunoreactivities (n = 3). Groups are indicated as control group (negative) and SOCS-1 shRNA group. **P < 0.01 as compared with negative group. EBP, enhancer binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PPAR, peroxisome proliferator-activated receptor; shRNA, small hairpin RNA; SOCS, suppressor of cytokine signaling.
Downregulation of SOCS-3 Expression Reduces the C/EBPα and PPARγ mRNA and Protein Level in Adipose Tissue
The relationship between SOCS-3 and the C/EBPα, the expression of PPARγ in adipose tissue, was also investigated by the same method as mentioned above. The result shows the same effect of SOCS-1 and SOCS-3 gene downregulation on C/EBPα, the expression of PPARγ. As compared with the control group, the mRNA expression of C/EBPα and PPARγ was significantly reduced (1.50 ± 0.47 vs. 2.27 ± 0.59, P < 0.05; 1.83 ± 0.88 vs. 4.33 ± 0.78, P < 0.01; Table 1 ) and the protein level of C/EBPα and PPARγ at 3 d after differentiation induction was also significantly reduced following the SOCS-3 gene silencing (P < 0.05; Figure 4 ).
Downregulation of SOCS-3 expression reduces the C/EBPα and PPARγ mRNA and protein level in adipose tissue in the IUGR rats at 3 d after differentiation. (a) Crude adipocyte extracts (50 μg/lane) were analyzed by western blots developed with antibodies to C/EBPα and PPARγ; GAPDH blot was included as a loading control. (b) Blots as shown in (a) were quantitated densitometrically and for quantitation of C/EBPα and PPARγ. The black bar refers to treatment with SOCS-3 shRNA, whereas the white bar indicates negative control. Data are presented as mean ± SD of the relative immunoreactivities (n = 3). Groups are indicated as control group (negative) and SOCS-3 shRNA group. *P < 0.05, **P < 0.01 as compared with negative group. EBP, enhancer binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IUGR, intrauterine growth retardation; PPAR, peroxisome proliferator-activated receptor; shRNA, small hairpin RNA; SOCS, suppressor of cytokine signaling.
Discussion
In clinical practice, the rapid deposition of adipose tissues has been noted in IUGR subjects, resulting in obesity and insulin resistance. The Third National Health and Nutrition Examination Survey in the United States revealed that the body weight of small-for-gestational-age subjects was still lower than that of normal subjects although sufficient nutrition had been administered. In addition, small-for-gestational-age subjects had high proportions of adipose tissue, and the amount of subcutaneous adipose tissue and hip circumference were larger than those in normal subjects (11). Our previous studies showed that the IUGR rats had a higher BMI as compared with the control group (4).
The molecular mechanisms underlying the regulation of adipocyte differentiation have been extensively studied in recent years. Many molecules are involved in the differentiation of preadipocytes into mature adipocytes. C/EBPα and PPARγ play important roles among these molecules: PPARγ can induce the maturation of adipocytes and regulate the expression of adipogenesis-related genes, whereas C/EBPα can regulate the terminal differentiation of adipocytes (12,13,14,15). Thus, the overdifferentiation of adipocytes and abnormal deposition of adipose tissues might be related to the increased expression of C/EBPα and PPARγ. On the basis of the results of the current study, the expression of C/EBPα and PPARγ as measured in an animal model demonstrated the above-mentioned hypothesis.
Given that C/EBPα and PPARγ can promote the maturation of adipocytes, leading to obesity, we investigated obesity in the current study. As is well known, insulin can promote adipogenesis and inhibit the degradation of adipose tissues. At the late stage of IUGR, insulin resistance and hyperinsulinemia may develop. Although the development of insulin resistance involves multiple molecules and multiple signaling pathways, the specific mechanisms for insulin resistance development are still poorly understood (16,17,18). It has been demonstrated that SOCS-1 and SOCS-3 can promote the development of insulin resistance (5,6,7), and obese subjects with insulin resistance have been found to have increased expression of SOCS-1 and SOCS-3 (5,14). Thus, we examined the expression of SOCS-1 and SOCS-3 in the subcutaneous adipose tissues of catch-up IUGR rats, and the results demonstrated increased expression of SOCS-1 and SOCS-3 as compared with normal rats. It is possible that increased expression of SOCS-1 and SOCS-3 can induce insulin resistance, leading to hyperinsulinemia, which facilitates the formation and deposition of adipose tissues. Alternatively, SOCS-1 and SOCS-3 may directly promote the expression of PPARγ and C/EBPα, resulting in adipogenesis and obesity.
To investigate these hypotheses, we designed shRNAs targeting SOCS-1 and SOCS-3, which were used to silence the expression of SOCS-1 and SOCS-3 during the differentiation of preadipocytes. We then measured the expression of PPARγ and C/EBPα. Induction of preadipocyte differentiation with a cocktail of steroids is a classic method that has high efficiency and can be completed within 1 wk. As shown in the study by Ntambi and Young-Cheul (13), the expression of C/EBPα and PPARγ reached a high level 3 d after differentiation induction. Therefore, we measured the expression of C/EBPα and PPARγ at this same time point. Our results showed that there was positive correlation between silencing of the SOCS-1 and SOCS-3 genes and the expression of C/EBPα and PPARγ, and that silencing of the SOCS-1 and SOCS-3 genes could significantly reduce the expression of C/EBPα and PPARγ. Thus, we speculate that abnormally increased expression of SOCS-1 and SOCS-3 promote the expression of C/EBPα and PPARγ in the adipose tissues, facilitating the formation and deposition of adipose tissues. In the current investigation, we downregulated the gene expressions of SOCS-1 and SOCS-3 in preadipocytes and then induced them in mature cells. At 72 h after differentiation, we determined the expressions of C/EBPα and PPARγ, two key molecules in the differentiation of preadipocytes. After transfecting the most effective shRNA recombinant plasmids into IUGR adipocytes, not only was a significant inhibition in SOCS-1 and SOCS-3 at both the mRNA and protein levels detected, but a reduction effect on the expression of C/EBPα and PPARγ was also detected.
Collectively, our results demonstrated that SOCS-1, SOCS-3, C/EBPα, and PPARγ expression are markedly increased in IUGR rats. During the differentiation of preadipocytes, silencing of SOCS-1 and SOCS-3 genes by using shRNAs can significantly reduce the expression of C/EBPα and PPARγ. These findings suggest that SOCS-1 and SOCS-3 are directly involved in the deposition of adipose tissues and even the development of obesity.
Methods
Animal Model and Preparation of Plasmids
During pregnancy, the rats were deprived of nutrition, but sufficient nutrition was administered to neonatal rats with the aim of establishing a catch-up IUGR rat model. Levels of fasting glucose and insulin were measured at 12 wk after birth to confirm the presence of obesity and insulin resistance. Four shRNAs targeting the rat SOCS-1 and SOCS-3 genes were designed, which include the U6 promoter and green fluorescent protein. Then the shRNA pair with the most effective silencing ability was selected. Details are shown in our previous study (4).
All experimental procedures were approved by the Center of Experimental Animals, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Culture of Preadipocytes
Preadipocytes were cultured according to the methods of Rodbell (19) and Yu and Eriksson (20); in brief, subcutaneous and perirenal white adipose tissues were collected and blood was removed by washing. Adipose tissues were then mixed with five volumes of 0.1% type I collagenase (Gibco, Grand Island, NY), followed by incubation at 37 °C for 45 min under continuous shaking for digestion. The mixture containing the cells was filtered through a 200-μm mesh filter, and the filtrate was collected into a centrifuge tube followed by centrifugation at 2,000 rpm for 10 min at 4 °C. These procedures were performed three times and the cells were counted. The cell density was adjusted to 1 × 105 cells/cm2, and then cells were seeded into six-well plates and maintained in Dulbecco’s modified essential medium–low glucose (Gibco) containing 10% fetal bovine serum (Gibco) at 37 °C in an atmosphere of 5% CO2.
Transfection of Adipocytes With Plasmids Carrying shRNAs
Preadipocytes were passaged three times and then seeded into six-well plates (4–5 × 104 cells/well) and maintained in antibiotic-free Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT). When the cell confluence reached 70%, transfection was performed. The preparation of shRNA-Lipofectamine 2000 (Invitrogen, Carlsbad, CA) compound was as follows: (i) 250 μl of Opti-MEM I (Invitrogen) was mixed with 5 μl of Lipofectamine 2000, followed by incubation at room temperature for 5 min; (ii) 250 μl of Opti-MEM I was mixed with 7.5 μl of shRNA, followed by incubation at room temperature for 5 min; and (iii) both mixtures were mixed gently, followed by incubation at room temperature for 20 min. The shRNA-Lipofectamine 2000 compound was added to a well containing cells and medium, followed by gentle shaking. The mixture was incubated at 37 °C in an atmosphere of 5% CO2 for 12 h, followed by induction of differentiation and maturation.
Differentiation Induction of Preadipocytes
At 24 h after transfection, a cocktail of steroids was used for differentiation induction of preadipocytes (21). Cells were incubated with Dulbecco’s modified essential medium containing 0.5 mmol/l 3-isobutyl-1-methylxanthine (Sigma-Aldrich, St Louis, MO), 1.0 μmol/l dexamethasone (Sigma-Aldrich), 10 μg/l insulin (Sigma-Aldrich), and 10% fetal bovine serum for 48 h, and the medium was refreshed with normal Dulbecco’s modified essential medium containing 10% fetal bovine serum for another 24 h. Cells were then harvested for subsequent detection.
Western Blotting Analysis
Proteins were extracted from subcutaneous and perirenal adipose tissues or cells with a protein extraction kit (Beyotime, Nantong, China) according to the manufacturer’s instructions. The bicinchoninic acid method (Beyotime, Nantong, China) was used to measure protein concentrations. Proteins (50 μg per lane) were subjected to electrophoresis and transferred onto a nitrocellulose membrane, which was then blocked in 5% nonfat milk for 2 h. Membranes were incubated with primary antibodies (dilutions: goat polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for SOCS-1 and SOCS-3, 1:400; polyclonal antibodies for C/EBPα, 1:200; rabbit polyclonal antibody (Santa Cruz Biotechnology) for PPARγ, 1:200 and for glyceraldehyde 3-phosphate dehydrogenase, 1:1,000) overnight at 4 °C. The membranes were washed with 0.1% PBS-Tween solution and then incubated with secondary horseradish peroxidase-conjugated antibody (1:4,000 dilution, Oncogene, Boston, MA) at room temperature for 2 h. Visualization was performed with the ECL kit (Thermo Scientific, Rockford, IL). Protein bands were collected by scanning with a UVP BioSpectrum imaging system (UVP, Upland, CA), and optical density was determined as the relative amount of proteins. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. Each experiment was performed in duplicate.
Detection of C/EBP-α and PPAR-γ mRNA Expression by Real-Time PCR
At 72 h after differentiation was induced, cells of the two groups were harvested, and total RNA was extracted using Trizol reagents (Invitrogen), followed by measurement of absorbance at 260 and 280 nm for the detection of RNA purity. The ratio of A260/A280 between 1.8 and 2 was acceptable. RNA was then reverse-transcribed into cDNA by using the RevertAid H minus first-strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania) on an ABI 7500 real-time PCR system (Applied Biosystems, Carlsbad, CA), followed by real-time PCR. The sequences of primers (Shanghai Sangon Bio-engineering, Shanghai, China) for PPARγ, C/EBPα, and β-actin were forward: 5′-ACCACAGTTGATTTCTCCAG-3′, reverse: 5′-TGTTGTAGAGCTGGGTCTTT-3′; forward: 5′-CGACTTCTACGAGGTGGAG-3′, reverse: 5′-ATGTAGGCGCTGATGTCTAT-3′; and forward: 5′-GGCATCCATGAAACTATT-3′, reverse: 5′-GATCTTCATGGTGCTAGGAG-3′, respectively; the corresponding anticipated sizes of these primers were 178, 192, and 165 bp, respectively. The mixture (25 μl) for PCR included 1 μl of template, 10 μmol/l primers (1 μl for each), 12.5 μl of SYBR Premix Ex Taq, and ddH2O (Ferments, Vilnius, Lithuania). Conditions for PCR were as follows: predenaturation at 95 °C for 5 min, 40 cycles of denaturation at 94 °C for 20 s, annealing at 58 °C for 20 s and extension at 72 °C for 20 s, and a final extension at 72 °C for 5 min. The comparative threshold cycle 2−ΔΔCT method was used for the determination of expressions of target genes.
Statistical Analysis
Data were expressed as mean ± SD. Comparisons between two groups were conducted using t-tests. SPSS version 15.0 statistics software (SPSS, Chicago, IL) was used for statistical analysis. A value of P < 0.05 was considered statistically significant.
Statement of Financial Support
This study was supported by the National Natural Science Foundation of China (grants 30672262, 30772358, and 81170627), PCSIRT1131, and the National Key Science Projects Program of the Ministry of Science and Technology (grant 2012BAI09B04).
References
Barker DJ, Eriksson JG, Forsén T, Osmond C . Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31:1235–9.
Kaijser M, Bonamy AK, Akre O, et al. Perinatal risk factors for diabetes in later life. Diabetes 2009;58:523–6.
Pallotto EK, Kilbride HW . Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol 2006;49:257–69.
Liao L, Zheng R, Wang C, et al. The influence of down-regulation of suppressor of cellular signaling proteins by RNAi on glucose transport of intrauterine growth retardation rats. Pediatr Res 2011;69:497–503.
Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E . SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 2000;275:15985–91.
Ueki K, Kondo T, Kahn CR . Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 2004;24:5434–46.
Rui L, Yuan M, Frantz D, Shoelson S, White MF . SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 2002;277:42394–8.
Gueerr-Millo M . Adipose tissue and adipokines: for better or worse. Diabetes Metab 2004;30:13–9.
Gumel M . Peroxisome proliferator-activated receptor gamma and the regulation of adipocyte function: lessons from human genetic studies. Best Pract Res Clin Endocrinol Metab 2005;19:501–23.
Qiao L, Maclean PS, Schaack J, et al. C/EBPα regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 2005;54:1744–54.
Hediger ML, Overpeck MD, Maurer KR, Kuczmarski RJ, McGlynn A, Davis WW . Growth of infants and young children born small or large for gestational age: findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med 1998;152:1225–31.
Tamori Y, Masugi J, Nishino N, Kasuga M . Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 2002;51:2045–55.
Ntambi JM, Young-Cheul K . Adipocyte differentiation and gene expression. J Nutr 2000;130:3122S–6S.
Peraldi P, Filloux C, Emanuelli B, Hilton DJ, Van Obberghen E . Insulin induces suppressor of cytokine signaling-3 tyrosine phosphorylation through janus-activated kinase. J Biol Chem 2001;276:24614–20.
Ahluwalia M, Evans M, Morris K, et al. The influence of the Pro12Ala mutation of the PPAR-γ receptor gene on metabolic and clinical characteristics in treatment-naïve patients with type 2 diabetes. Diabetes Obes Metab 2002;4:376–8.
Enriori PJ, Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 2007;5:181–94.
Lönnqvist F, Arner P, Nordfors L, Schalling M . Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med 1995;1:950–3.
Kondo H, Shimomura I, Matsukawa Y, et al. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes 2002;51:2325–8.
Rodbell M . Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964;239:375–80.
Yu ZW, Eriksson JW . The upregulating effect of insulin and vanadate on cell surface insulin receptors in rat adipocytes is modulated by glucose and energy availability. Horm Metab Res 2000;32:310–5.
Otto TC, Lane MD . Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 2005;40:229–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, RD., Liao, LH., Ye, J. et al. Effects of SOCS 1/3 gene silencing on the expression of C/EBPα and PPARγ during differentiation and maturation of rat preadipocytes. Pediatr Res 73, 263–267 (2013). https://doi.org/10.1038/pr.2012.190
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.190
This article is cited by
-
LRP6 Bidirectionally Regulates Insulin Sensitivity through Insulin Receptor and S6K Signaling in Rats with CG-IUGR
Current Medical Science (2023)
-
IUGR with infantile overnutrition programs an insulin-resistant phenotype through DNA methylation of peroxisome proliferator–activated receptor-γ coactivator-1α in rats
Pediatric Research (2015)
-
Suppressors of cytokine signaling (SOCS) and type 2 diabetes
Molecular Biology Reports (2014)