Abstract
Background:
Kawasaki disease (KD) can result in fatal coronary artery (CA) aneurysms, especially if left untreated. Our recent studies of its vascular pathology revealed subacute/chronic vasculitis that begins early in the illness with the proliferation of smooth muscle cell–derived myofibroblasts in a complex extracellular matrix (ECM). We hypothesized that a dysregulation of specific ECM and adhesion molecules occurs in KD CAs.
Methods:
Gene expression profiling for ECM and adhesion molecules was performed on six acute KD and eight control CAs using a targeted real-time PCR array approach.
Results:
Integrins α4 and αM (ITGA4, ITGAM), collagen type I, α1 (COL1A1), and matrix metalloproteinase 7 (MMP7) were significantly upregulated in KD CAs as compared with controls. Immunohistochemistry with anti-ITGAM antibodies revealed expression on inflammatory cells within the CA wall in patients with KD but not in controls.
Conclusion:
Integrins ITGA4 and ITGAM are upregulated in KD vasculopathy, probably promoting inflammatory recruitment that stimulates smooth muscle cell transition to myofibroblasts and their proliferation. MMP7 probably enhances myofibroblast proliferation and luminal lesion expansion, and overexpression of COL1A1 may lead to CA stenosis. Identification of the molecular pathogenesis of KD vasculopathy may lead to the development of circulating biomarkers and to directed therapeutic interventions.
Similar content being viewed by others
Main
Gene expression profiling is a powerful tool in the study of disease pathogenesis, the development of biomarkers and prognostic indicators, and the identification of new therapeutic targets. Examples of its clinical application in cardiovascular disease include peripheral blood gene expression profiling to identify individuals at low risk of acute rejection following cardiac transplantation, thereby reducing the need for biopsies (1,2). Expression profiling of myocardial biopsies in patients with cardiomyopathy has led to the identification of disease-specific expression profiles (3,4).
The molecular pathogenesis of coronary artery (CA) abnormalities in Kawasaki disease (KD), the leading cause of acquired heart disease in children in developed countries, is unknown, and gene expression profiling of acute KD CA has never been performed. We recently analyzed vascular pathology in 41 KD cases and identified three linked pathologic processes in KD vasculopathy (5). Necrotizing arteritis, the first process, is an acute self-limited neutrophilic process beginning and ending within the first 2 wk of fever onset, progressively destroying the vessel wall from the endothelium into the adventitia and causing saccular aneurysms that can thrombose or rupture. This process is complete within 2 wk of illness onset. Two other processes are also ongoing in the first 2 wk, can continue indefinitely, and are probably particularly important in those patients with KD who do not respond to intravenous immunoglobulin therapy and continue to have progressive CA dilatation: subacute/chronic vasculitis, which consists of mostly small lymphocytes but also plasma cells and eosinophils with some macrophages, and luminal myofibroblastic proliferation (LMP), a progressive stenosing intraluminal proliferative lesion whose pathognomonic cell is a smooth muscle cell–derived myofibroblast, and its matrix products. These pathologic findings strongly suggest an important role for extracellular matrix (ECM) molecules in the pathophysiology of CA lesions in KD. Therefore, we hypothesized that specific ECM and cell adhesion molecules are dysregulated in acute KD CA as compared with childhood control CAs. We used gene expression profiling and immunohistochemistry to examine these genes and protein expression, respectively, in KD and control CA tissues.
Results
Clinical and Pathologic Findings in KD and Control Patients
The clinical and pathologic data on the KD and control patient tissues used in this study are provided in Tables 1 and 2 . All patients with KD had severe CA disease and died in the acute phase of illness (within 5 wk of fever onset); pathologic examination showed subacute/chronic vasculitis and LMP (5). Control CA tissues were obtained from children who died of non-KD illnesses and had normal CA histology.
Evaluating ECM and Adhesion Molecule Gene Expression
RNA isolated from six KD and eight control CA specimens were of adequate quality for PCR array analyses. The gene expression was normalized to the housekeeping gene HPRT1, and relative gene expression was evaluated in KD as compared with control CA. Of 84 genes involved in cell–cell and cell–matrix interactions on the array, four were found to be statistically significantly upregulated in the KD specimens: integrin α4 (ITGA4), integrin αM (ITGAM), collagen, type I, α1 (COL1A1), and matrix metalloproteinase 7 (MMP7) ( Table 3 ). Thus, array profiling of KD tissue revealed dysregulation of genes involved in ECM gene expression (COL1A1 and MMP7) and adhesion molecules (integrins αM and α4). These genes clustered with KD diagnosis ( Figure 1 ); this result was irrespective of KD therapy ( Table 1 ).
Heat map of genes whose expression was significantly different between coronary arteries of patients with KD and those of controls. There was clustering of KD samples, irrespective of therapy. COL1A1, collagen, type I, α1; ITGA4, integrin α4; ITGAM, integrin αM; KD, Kawasaki disease; MMP7, matrix metalloproteinase 7.
Expression of Integrin αM Protein in KD CA Tissue
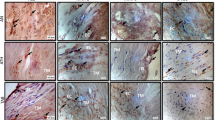
To determine if protein expression of integrin αM was also upregulated, we performed immunohistochemistry on six KD and five control CA tissues. We found that expression of ITGAM was observed in all six KD but none of the five control CA tissues ( Figure 2 ). Integrin αM was expressed on inflammatory cells within KD tissues, predominantly macrophages ( Figure 2 ). It was also expressed on spindle-shaped cells in LMP lesions ( Figure 2c ).
Histology and immunohistochemistry (IHC) of coronary arteries (CAs) of patients with Kawasaki disease (KD) and childhood control. (a) Childhood control CA is free of inflammation and luminal proliferation and has a thin intima covering an undulating elastic lamina (arrow) and a uniform media. Hematoxylin and eosin stain (H&E), ×10 objective, bar = 200 µm. (b) Portion of KD CA with luminal subacute/chronic vasculitis–luminal myofibroblastic proliferation (SA/C-LMP) (long arrows) of varying thickness. The underlying internal elastic lamina (short arrow) is mostly intact, whereas the media (M) is free of inflammation and somewhat tangentially sectioned. A small portion of visible adventitia in the lower right has SA/C inflammation. H&E stain, ×10 objective, bar = 200 µm. (c) IHC for integrin αM (ITGAM) reveals positive cells (brown) in the adventitia (ADV), SA/C-LMP, and lumen of a damaged KD CA. ×10 objective, bar = 200 µm. (d) At higher magnification, the positive cells can be seen to be both spindle-shaped (long arrows) and mononuclear inflammatory cells (short arrows). ×40 Objective, bar = 50 µm. (e) Childhood control CA, no ITGAM expression in the arterial wall. A few positive-staining circulating mononuclear cells are present in the lumen (arrows). IHC for ITGAM, ×20 objective, bar = 50 µm. (f) A section of a small artery located in KD CA periadventitial tissue is almost filled with ITGAM-positive mononuclear cells. IHC for ITGAM, ×20 objective, bar = 50 µm.
Discussion
Our recent pathologic study of vascular tissues from 41 KD cases demonstrated that smooth muscle cell–derived myofibroblasts actively proliferate in an uncontrolled fashion in the KD arterial wall and secrete or shed an active ECM. The proliferative LMP process can progress to life-threatening CA occlusion and myocardial ischemia over months to years (5). Although therapies are available to reduce thrombosis in KD vascular tissues, no therapy is available to reduce LMP. Given that the vast majority of KD fatalities occur after the second week of illness, when subacute/chronic vasculitis and LMP are the active processes in the vascular wall, the pathophysiology of the processes must be better understood in order to develop new preventative and therapeutic regimens. Here, we used expression profiling and report that four genes, ITGA4, MMP7, ITGAM, and COL1A1, were significantly upregulated in KD CA specimens. Only one prior study of gene expression profiling of KD CA tissues has been performed; this study included late-stage KD tissues only and did not focus on ECM and adhesion molecules (6).
The integrins are a family of receptors for ECM and cell surface ligands involved in cell migration and attachment to the ECM; they are composed of α and β subunits. Bound integrins can both transmit and receive intracellular signals, thereby regulating endothelial cell migration and survival as well as angiogenesis, linking components of the ECM, and modulating cellular proliferation, adhesion, and motility (7,8,9). We identified upregulation of ITGAM in patients with KD; ITGAM is expressed on dendritic cells, macrophages, monocytes, and neutrophils when complexed with β2 integrin (10). ITGAM has multiple ligands and is involved in the regulation of neutrophil and monocyte adhesion and migration to damaged endothelium and endothelial-associated ECM. We also noted expression of ITGAM on spindle-shaped cells within KD LMP lesions, some of which may be myofibroblasts; we previously showed by transmission electron microscopy that the cellular component of LMP was a smooth muscle cell–derived myofibroblast (5). In this regard, two animal studies are of particular interest. ITGAM knockout mice subjected to endothelial denudation and arterial stretching were noted to have reduced intimal thickening and cell proliferation, as well as reduced leukocyte accumulation as compared with control mice (11). Another murine model of vascular injury demonstrated decreased inflammatory cell infiltrate and reduced long-term intimal thickening in the presence of anti-αM antibodies (12). Of note, ITGAM was reported to be upregulated in the peripheral blood of patients with KD who were refractory to initial therapy (13).
ITGA4 (integrin α4) was also noted to be upregulated in KD CA tissues. Expression of ITGA4 on peripheral blood leukocytes facilitates rolling and adhesion to activated endothelium and is thus implicated in inflammatory recruitment (14,15). ITGA4 is also expressed on endothelial cells and binds to fibronectin and vascular cell adhesion molecule 1, promoting tumor lymphangiogenesis induced by vascular endothelial growth factor–A and –B (16). ITGA4 has been noted to be upregulated in abdominal aortic aneurysms (17) and in the peripheral blood mononuclear cells of cardiac transplant patients with acute rejection (18). In KD CA lesions, ITGAM and ITGA4 are likely to promote subacute/chronic vasculitis, leading to smooth muscle cell transition to myofibroblasts and their proliferation. Integrins have recently served as a therapeutic target in inflammatory diseases such as multiple sclerosis and Crohn’s disease; natalizumab (Tysabri), an anti- ITGA4 monoclonal antibody, has been approved by the US Food and Drug Administration for the treatment of both conditions.
COL1A1 is an interstitial matrix molecule found in most connective tissues and involved in wound healing and remodeling. Transforming growth factors β1, β2, and β3, platelet-derived growth factor, interleukin (IL)-1α, IL-1β, and IL-4, and mast cell tryptase increase production of type I collagen (19). IL-1-related genes are upregulated in KD peripheral blood during the acute phase of illness (20), and a murine model demonstrates a critical role for IL-1β in the development of vasculitis (21). Upregulation of type I collagen has been observed in injured vascular media (22), and excessive collagen production probably contributes to CA stenosis in more severe KD cases.
MMP7 (matrilysin) is a matrix metalloproteinase that is involved in the breakdown of ECM proteins, including proteoglycans, elastin, laminin, fibronectin, gelatin, and entactin. Monocytes and macrophages have been noted to produce MMP7 in response to severe inflammation (23), and MMP7 is known to play a role in inflammatory recruitment of neutrophils via a chemotactic gradient (24). MMP7 has protective functions, including a role in innate immunity in gut mucosal tissues and wound healing (25). However, MMP7 has also been implicated in pathologic processes such as pulmonary fibrosis, the development and progression of certain cancers, and promotion of thrombosis and plaque rupture in coronary atherosclerosis (26). Notably, increased plasma levels of MMP7 have been demonstrated in patients with CA disease, metastatic colon and rectal cancer, lung cancer, and pancreatic cancer, suggesting its possible utility as a biomarker (27,28,29,30,31). Promising research is currently ongoing using matrix metalloproteinase inhibitors as therapeutic interventions for a variety of cancers (31). Future studies will focus on MMP7 as a possible prognostic and diagnostic biomarker in KD sera.
Our study has several limitations. Because KD fatalities are not reportable, and deaths are scattered, the number of tissue specimens available for study are limited. Two of our patients were untreated, but four others received several therapies that could have affected gene expression. However, in this small sample of patients, differences in therapy did not appear to significantly alter the expression of the four upregulated genes ( Figure 1 ; Table 1 ). This may be because current therapies do not target expression of adhesion molecules and ECM proteins or because these patients were so severely affected that therapeutic intervention was not efficacious. Although control children in this study had pathologically normal CAs, it is possible that their non-KD illnesses affected gene expression in the CAs. This study was designed to examine the ECM and cell adhesion molecules using a commercially available array, and therefore, was restricted to only those 84 genes on the array.
In conclusion, gene expression analysis of acute KD CA vasculopathy reveals upregulation of ITGA4, ITGAM, COL1A1, and MMP7, and immunohistochemistry revealed strong expression of ITGAM in inflammatory cells of KD CAs. Determining molecular events in the KD arterial wall is key to rational drug design of urgently needed new therapies for patients with KD who do not respond to infusion of intravenous immunoglobulin, and secreted molecules that are upregulated in KD arterial tissues should be studied as possible circulating disease biomarkers.
Methods
Tissues
KD and control CA tissues were deidentified autopsy samples; therefore, institutional review board approval and informed consent were not required because the study did not meet the criteria of human subjects research as defined by the US Department of Health and Human Services.
RNA Extraction From Formalin-Fixed Paraffin-Embedded Tissues
RNA was extracted from formalin-fixed paraffin-embedded tissue sections (7–10 µm) of CA from nine patients with KD and 11 pediatric controls using the Qiagen RNeasy FFPE Kit (Qiagen/SA Biosciences, Valencia, CA) per manufacturer’s instructions, except that proteinase K lysis was performed for 1 h at 56 °C. RNA quantity was measured using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE).
cDNA Synthesis and cDNA Quality Assessment
Single-strand cDNA was synthesized from 300 ng of extracted RNA using the Qiagen/SA Biosciences RT2 preAMP cDNA Synthesis Kit according to the manufacturer’s instructions. The quality of each cDNA sample was assessed in triplicate by real-time PCR using SYBR Green chemistry and primers for the RNA housekeeping gene RPL13A and for human genomic DNA contamination (Qiagen/SA Biosciences). Samples were considered to be of good quality if the C(t) (threshold cycle) values for human genomic DNA contamination were at least three higher (eightfold-change) than for RPL13A and the RPL13A C(t) was <35.
ECM and Adhesion Molecule Array
Those cDNA samples that passed quality control underwent preamplification with array-specific primers using the Qiagen/SA Biosciences RT2 preAMP Pathway Primer Mix according to the manufacturer’s instructions and were applied to the ECM and adhesion molecule array plate (Qiagen/SA Biosciences PAHS-013). Using a CFX96 X real-time PCR detection system (Biorad, Hercules, CA), the following cycling program was used: 95 °C for 10 min, then 40 cycles of 95 °C for 15 s, and 60 °C for 1 min, then 95 °C for 10 s with a melting curve performed from 65 to 95 °C, with increments of 0.5 °C every 5 s.
Immunohistochemistry
Immunohistochemistry was performed on KD and control CA formalin-fixed paraffin-embedded tissue sections as previously described (32,33) for ITGAM (Prestige Antibodies, 1:300; Sigma, St. Louis, MO). Briefly, antigen retrieval was performed in 0.01 mol/l sodium citrate buffer at a pH of 6.0 using a pressure cooker. The Vectastain Elite ABC system (Vector, Burlingame, CA) was used with diaminobenzidine as the chromagen to yield a brown color.
Statistical Analysis
We compared the expression levels of each gene between KD and controls by calculating ΔC(t) values. Fold changes were calculated by the Δ–ΔC(t) method. Using ΔC(t) values, comparisons were made for each gene using t-tests. Multiple comparisons were accounted for by calculating the false discovery rate using q values (34,35,36). Furthermore, if ≥50% of either KD or control samples had undetected/undetermined C(t) values for a specific gene, then that gene was excluded from the study. For hierarchical clustering analysis, we used Euclidean distance as a metric for dissimilarity and summarized the results as a heat map.
Statement of Financial Support
This work was supported by National Institute of Health grants HL63771 and HL109955 to A.H.R., the Max Goldenberg Foundation, the Kawasaki Disease Fund, and the Center for Kawasaki Disease at the Ann and Robert H. Lurie Children’s Hospital of Chicago.
References
Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med 2010;362:1890–900.
Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant 2006;6:150–60.
Kittleson MM, Minhas KM, Irizarry RA, et al. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics 2005;21:299–307.
Tan FL, Moravec CS, Li J, et al. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci USA 2002;99:11387–92.
Orenstein JM, Shulman ST, Fox LM, et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS ONE 2012;7:e38998.
Fukazawa R, Ikegam E, Watanabe M, et al. Coronary artery aneurysm induced by Kawasaki disease in children show features typical senescence. Circ J 2007;71:709–15.
Takada Y, Ye X, Simon S . The integrins. Genome Biol 2007;8:215.
Avraamides CJ, Garmy-Susini B, Varner JA . Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 2008;8:604–17.
Cantor JM, Ginsberg MH, Rose DM . Integrin-associated proteins as potential therapeutic targets. Immunol Rev 2008;223:236–51.
Hom G, Graham RR, Modrek B, et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med 2008;358:900–9.
Simon DI, Dhen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C . Decreased neointimal formation in Mac-1(-/-) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest 2000;105:293–300.
Wang Y, Sakuma M, Chen Z, et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation 2005;112:2993–3000.
Abe J, Ebata R, Jibiki T, Yasukawa K, Saito H, Terai M . Elevated granulocyte colony-stimulating factor levels predict treatment failure in patients with Kawasaki disease. J Allergy Clin Immunol 2008;122:1008–1013.e8.
Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA . P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol 1997;159:3929–39.
Johnston B, Issekutz TB, Kubes P . The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med 1996;183:1995–2006.
Garmy-Susini B, Avraamides CJ, Schmid MC, et al. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res 2010;70:3042–51.
Lenk GM, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H . Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genomics 2007;8:237.
Bernstein D, Williams GE, Eisen H, et al. Gene expression profiling distinguishes a molecular signature for grade 1B mild acute cellular rejection in cardiac allograft recipients. J Heart Lung Transplant 2007;26:1270–80.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002;420:860–7.
Popper SJ, Shimizu C, Shike H, et al. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol 2007;8:R261.
Lee Y, Schulte DJ, Shimada K, et al. Interleukin-1ß is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation 2012;125:1542–50.
Newby AC, Zaltsman AB . Molecular mechanisms in intimal hyperplasia. J Pathol 2000;190:300–9.
Busiek DF, Baragi V, Nehring LC, Parks WC, Welgus HG . Matrilysin expression by human mononuclear phagocytes and its regulation by cytokines and hormones. J Immunol 1995;154:6484–91.
Swee M, Wilson CL, Wang Y, McGuire JK, Parks WC . Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol 2008;83:1404–12.
Parks WC, Wilson CL, López-Boado YS . Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–29.
Wielockx B, Libert C, Wilson C . Matrilysin (matrix metalloproteinase-7): a new promising drug target in cancer and inflammation? Cytokine Growth Factor Rev 2004;15:111–5.
Nilsson L, Jonasson L, Nijm J, Hamsten A, Eriksson P . Increased plasma concentration of matrix metalloproteinase-7 in patients with coronary artery disease. Clin Chem 2006;52:1522–7.
Bäck M, Ketelhuth DF, Agewall S . Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis 2010;52:410–28.
Kurokawa S, Arimura Y, Yamamoto H, et al. Tumour matrilysin expression predicts metastatic potential of stage I (pT1) colon and rectal cancers. Gut 2005;54:1751–8.
Kuhlmann KF, van Till JW, Boermeester MA, et al. Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2007;16:886–91.
Roy R, Yang J, Moses MA . Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol 2009;27:5287–97.
Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH . CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis 2001;184:940–3.
Gavin PJ, Crawford SE, Shulman ST, Garcia FL, Rowley AH . Systemic arterial expression of matrix metalloproteinases 2 and 9 in acute Kawasaki disease. Arterioscler Thromb Vasc Biol 2003;23:576–81.
Storey JD . A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol 2002;64:479–98.
Storey JD, Taylor JE, Siegmund D . Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc Series B Stat Methodol 2004;66:187–205.
Storey JD, Tibshirani R . Statistical significance for genomewide studies. Proc Natl Acad Sci USA 2003;100:9440–5.
Acknowledgements
We thank Robin Biggs, Deidre Anderson, and Leslie Martin for assistance in identifying and dissecting control CA samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reindel, R., Baker, S., Kim, KY. et al. Integrins α4 and αM, collagen1A1, and matrix metalloproteinase 7 are upregulated in acute Kawasaki disease vasculopathy. Pediatr Res 73, 332–336 (2013). https://doi.org/10.1038/pr.2012.185
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.185
This article is cited by
-
Bioinformatics analysis and identification of hub genes of neutrophils in Kawasaki disease: a pivotal study
Clinical Rheumatology (2023)
-
The Roles of Genetic Factors in Kawasaki Disease: A Systematic Review and Meta-analysis of Genetic Association Studies
Pediatric Cardiology (2018)
-
The transcriptional profile of coronary arteritis in Kawasaki disease
BMC Genomics (2015)
-
Kawasaki disease: insights into pathogenesis and approaches to treatment
Nature Reviews Rheumatology (2015)