Abstract
Background:
Neonatal hyperbilirubinemia arises from increased bilirubin production and decreased bilirubin elimination. Although phototherapy safely and effectively reduces bilirubin levels, recent evidence shows that it has adverse effects. Therefore, alternative treatments are warranted. Metalloporphyrins, competitive inhibitors of heme oxygenase (HO), the rate-limiting enzyme in bilirubin production, effectively reduce bilirubin formation; however, many are photoreactive. Here, we investigated possible photosensitizing effects of chromium mesoporphyrin (CrMP) and zinc deuteroporphyrin bis-glycol (ZnBG).
Methods and Results:
Administration of CrMP or ZnBG to 3-d-old mouse pups (3.75–30.0 µmol/kg intraperitoneally) and exposure to cool white (F20T12CW) and blue (TL20W/52) fluorescent lights (+L) for 3 h, resulted in a dose-dependent mortality (50% lethal dose (LD50) = 21.5 and 19.5 µmol/kg, respectively). In contrast to ZnBG, there was no significant difference in survival between the CrMP+L and CrMP groups. Following 30 µmol/kg ZnBG+L, we found significant weight loss, decreased liver antioxidant capacities, and increased aspartate aminotransaminase levels. At 6-d post-light exposure, ZnBG+L-treated pups showed gross and histologic skin changes at doses >7.5 µmol/kg. No lethality was observed following treatment with 30 µmol ZnBG/kg plus exposure to blue light-emitting diodes. Phototoxicity of ZnBG was dependent on light source, emission spectrum, and irradiance.
Conclusion:
Low doses of ZnBG (<3.75 µmol/kg) retained maximal HO inhibitory potency without photosensitizing effects, and therefore are potentially useful in treating neonatal hyperbilirubinemia.
Similar content being viewed by others
Main
Phototherapy is considered safe and effective for treating neonatal hyperbilirubinemia, and therefore protects infants against bilirubin-induced neurotoxicity. Since its introduction in the United States, there has been a dramatic reduction in severe hyperbilirubinemia and the need for exchange transfusions, a high-risk procedure with many possible adverse effects (1,2). Although overall reports about significant toxicity resulting from phototherapy are exceptionally rare, adverse effects have been observed in preterm newborns. Because the antioxidant capacity of preterm newborns is limited, phototherapy can further decrease defense mechanisms against reactive oxygen species through reducing bilirubin levels and through promoting photochemical reactions. Impaired anticoagulation, re-opening of the patent ductus arteriosus, and platelet destruction have been observed in infants undergoing phototherapy (3). A NICHD trial comparing aggressive vs. conservative phototherapy indicated that extremely low birth weight infants weighing 501–750 g are more susceptible to injurious effects of phototherapy than were infants in the 751–1,000 g cohort. Post hoc analyses revealed a significant decrease in the overall rate of neurodevelopmental impairment with aggressive phototherapy; this reduction was offset by an increased mortality in the 501–750 g cohort, which however, was not statistically significant (2). Moreover, phototherapy can affect the immune system via alterations in cytokine production (4). Therefore, identifying alternative treatment strategies primarily targeting high bilirubin producers in extremely low birth weight infants is needed.
In 1981, using metalloporphyrins (Mps) to inhibit bilirubin production was pioneered by Drummond and Kappas (5) and Maines (6). These synthetic heme analogs competitively inhibit heme oxygenase (HO), the enzyme that degrades heme to biliverdin, carbon monoxide (CO), and free iron (Fe2+) (7). Biliverdin is subsequently reduced to bilirubin by biliverdin reductase, and the iron-storage protein, ferritin, sequesters Fe2+ (8,9). Although many Mps have been shown to effectively inhibit bilirubin production, their clinical use has been viewed critically. Mps can affect nitric oxide synthase or soluble guanylyl cyclase (10,11), can increase gene expression and protein levels of the inducible HO-1 (12), and are potential photosensitizers (13,14,15). Tin protoporphyrin (SnPP) and tin mesoporphyrin (SnMP) have been studied in human neonates regarding their safety and efficacy (16,17), however, both showed photosensitizing effects in animal models (18), which led to the abandonment of SnPP. SnMP has minimal photosensitizing effects clinically, mainly because of its greater HO inhibitory potency and lower therapeutic dose (6 µmol/kg). Yet it strongly induces HO-1 gene and protein expression (19), has a long duration of action (19), passes the blood-brain barrier (unpublished data), inhibits soluble guanylyl cyclase (11), and contains a nonphysiological metal atom.
Recently, our studies have focused on chromium mesoporphyrin (CrMP) and zinc deuteroporphyrin bis-glycol (ZnBG). Both are potent inhibitors of HO, are orally absorbable, and have negligible effects on nitric oxide synthase and soluble guanylyl cyclase (10). Moreover, CrMP does not affect brain or spleen HO activity 24 h after oral administration (unpublished data), decreases liver HO activity in a hemolytic model (unpublished data), and is not photoreactive (20). We have shown that ZnBG is highly potent and fast-acting with a short duration of action, minimally increases HO-1 gene expression and protein levels, and decreases HO activity and bilirubin production in a hemolytic mouse model (19,21). Therefore, here, we evaluated the photoreactivity of CrMP and ZnBG in a 3-d-old mouse model under various light conditions.
Results
Photo- and/or Chemical Toxicity of CrMP and ZnBG
There were no significant differences between survival curves of CrMP and CrMP+L groups at any dose ( Figure 1a,b ). Fifty percent lethal doses (LD50s) were interpolated to be 23.0 and 21.5 µmol/kg for CrMP and CrMP+L, respectively. Survival percentages in the ZnBG groups were 100% at all doses ( Figure 1c ). Following light exposure, ZnBG+L showed a concentration-dependent phototoxicity with an LD50 of 19.5 µmol/kg ( Figure 1d ). Survival proportions of 15 and 30 µmol ZnBG/kg+L significantly differed from pups treated at equal doses without light exposure (P < 0.05 and P < 0.001, respectively). Because of the observed chemical toxicity of CrMP, further studies were performed using ZnBG only.
Survival curves of 3-d-old pups after intraperitoneal injection of vehicle (0, solid line), or 3.75 (dashed and dotted line), 7.5 (long dashed line), 15 (short dashed line), or 30 (dashed dotted dotted line) µmol/kg of (a) chromium mesoporphyrin (CrMP) or zinc deuteroporphyrin bis-glycol (ZnBG) (c) alone and after exposure to 2×cool white (CW)/1×blue fluorescent (BF) light at 35.0 ± 1.0 µW/cm2/nm for 3 h (CrMP+L and ZnBG+L, (b) and (d), respectively). No significant differences were observed between CrMP and CrMP+L groups for each dose (log-rank test). In contrast, significant differences in survival were found between ZnBG and ZnBG+L groups at 15 and 30 µmol/kg. Pups given ZnBG and kept in subdued light showed no toxic effects, whereas light exposure resulted in a concentration-dependent toxicity (*P < 0.05, **P < 0.001 vs. ZnBG at equal doses, log-rank test). n = 10–17 pups from at least three different litters were used in each group.
Physical Changes After ZnBG+L
Following ZnBG+L treatment, pups showed a dose-dependent weight loss, which was significant for 15 and 30 µmol ZnBG/kg ( Figure 2 , P < 0.001). Moreover, we observed discomfort during light exposure, lethargy near the end of and post-light treatment, and hypothermia, with increasing intensities for all signs listed in Table 1 associated with increasing ZnBG doses. At 15 and 30 µmol/kg ZnBG+L doses, signs of severe systemic toxicity (i.e., ataxia, hunched back, cyanosis, and a hypersensitivity to touch) ( Table 1 ) were observed. Survivors of different exposure setups (7.5, 15, and 30 µmol/kg) developed transient skin irritations at injection sites and on scalps, appearing 4–6-d post-light exposure, lasting for ~2 wk, and resolving 3-wk post-light exposure. Hematoxylin and eosin staining of the abdominal skin and scalp of pups treated with 30 µmol ZnBG/kg+L and exposed to 2×BF revealed epithelial hyperplasia with varying degrees of hyperkeratosis and parakeratosis, subepithelial expansion with severe inflammatory infiltration, and gross identifiable ulcerations 6-d post-light exposure ( Figure 3 ).
Weight loss after zinc deuteroporphyrin bis-glycol (ZnBG)+L treatment. 3-d-old pups treated with 15 and 30 µmol ZnBG/kg+L (2×cool white/1×blue fluorescent tubes) at 35.0 ± 1.0 µW/cm2/nm for 3 h had significant weight loss as compared with light-exposed vehicle (VEH)-treated pups (0+L). *P < 0.001 vs. VEH+L, one-way ANOVA, followed by Bonferroni’s multiple comparison test.
Histological changes in skin after zinc deuteroporphyrin bis-glycol (ZnBG)+L treatment. (a, b) Skin of 3-d-old vehicle (VEH)-treated pups, exposed to 2×blue fluorescent (BF) tubes at 35.0 ± 1.0 µW/cm2/nm for 3 h shows mild hyperplasia of the epidermal squamous epithelium (b, 0+L) compared to VEH-treated no light controls (a, 0). (c, d) Abdominal skin (c, 30+L) and scalp (d, 30+L) of 30 µmol ZnBG/kg-treated pups exposed to BF light for 3 h shows severe damage 6 d post-light exposure. (c) Abdominal skin reveals epithelial hyperplasia with irregular cord-like growths of epithelial cells into the dermis and varying degrees of hyperkeratosis and parakeratosis. (d) Scalp shows predominant inflammatory infiltration and ulceration. Upper and lower panels show sections at 200× and 400× original magnifications, respectively.
Liver Damage and Reduced Antioxidant Capacity Following ZnBG+L
Plasma aspartate aminotransaminase (AST) levels (marker of liver function) significantly increased 1.8-fold as compared with vehicle-treated (VEH) pups without light treatment. Surprisingly, alkaline phosphatase (ALP) activity significantly decreased by 50% in the ZnBG+L treatment group. A total of 3.75 µmol/kg ZnBG+L treatment did not affect AST values ( Figure 4a,b ). To assess the potential of liver tissue for oxidation via the Fenton reaction, CO production was measured as an index of lipid peroxidation (LP) and, therefore as an inverse index of tissue antioxidants (22). Post-light exposure, liver LP increased significantly after 30 µmol/kg ZnBG+L (90 ± 64 pmol CO/h/mg fresh weight (FW), n = 28, P < 0.001) as compared with VEH (7 ± 6 pmol CO/h/mg FW, n = 22, Figure 4c ). VEH+L and 3.75 µmol/kg ZnBG+L showed a similar, but not significant, elevation in LP (45 ± 42 pmol CO/h/mg FW, n = 12; 39 ± 44 pmol CO/h/mg FW, n = 9, respectively). Of note, intramuscular administration of 10 mg Vitamin E (Vit E)/kg 24 h before the light exposure completely abolished light-mediated increases in LP of VEH+L and ZnBG+L liver tissues (2 ± 1 pmol CO/h/mg FW, n = 3; 6 ± 4 pmol CO/h/mg FW, n = 6, respectively, P < 0.01).
Markers of tissue injury after zinc deuteroporphyrin bis-glycol (ZnBG)+L treatment. (a) Aspartate aminotransaminase (AST) and (b) alkaline phosphatase (ALP) activities after vehicle (VEH) (0) and ZnBG treatment of 3-d-old pups kept in subdued light (black bars) or exposed to 2×cool white (CW)/1×blue fluorescent (BF) (white bars). Pups treated with 30 µmol ZnBG/kg+L showed significant increases in AST levels, and decreases in ALP levels, as compared with VEH-treated mice, whereas pups treated with 3.75 µmol ZnBG/kg+L showed similar AST, and ALP levels to those of VEH-treated pups. Each data point represents pooled tissue (n = 3) in the same treatment group with 3–14 data points for each treatment group. *P < 0.01 vs. VEH, one-way ANOVA followed by Bonferroni’s multiple comparison test. (c) Antioxidant capacity of liver tissues after VEH (0), ZnBG, and VEH or ZnBG treatment plus pre-treatment with 10 mg vitamin E (Vit E)/kg (gray bars) of 3-d-old pups in subdued light (black bars), or exposed to 2×CW/1×BF light (white bars). Lipid peroxidation (LP) significantly increased in liver tissues of pups treated with 30 µmol ZnBG/kg+L as compared with VEH. Administration of 10 mg Vit E/kg intramuscularly, 24 h before ZnBG injection (ZnBG+L+Vit E), decreased LP and therefore increased liver antioxidant capacity up to VEH values. n ≥ 12 with and without ZnBG administration, n ≥ 3 for Vit E-treated groups. **P < 0.001 vs. VEH; †P < 0.01 vs. ZnBG+L, one-way ANOVA followed by Bonferroni’s multiple comparison test.
Effect of Different Light Sources
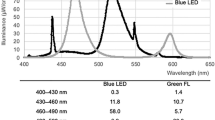
Using different exposure setups (cool white (CW), blue fluorescent (BF), blue light-emitting diode (BLED), or white LED light) ( Table 2 ), we investigated whether ZnBG-mediated phototoxicity was dependent on light source, emission spectrum, and/or irradiance. No significant differences in survival proportion were found between pups given 30 µmol ZnBG/kg and exposed to 2×BF or 2×CW-low irradiance (LI) tubes (n = 10, Figure 5a ) at 3,491 ± 80 and 1,991 ± 80 µW/cm2, respectively ( Figure 5b ). However, survival proportions of pups exposed to 5,148 ± 100 µW/cm2, 3×CW-high irradiance (HI) were significantly decreased (P < 0.0001) as compared with those exposed to 2×CW-LI ( Figure 5a,b ). Of note, 100% survival was observed after ZnBG administration and exposure to BLED (n = 13, Figure 5c ) at an irradiance of 2,250 ± 60 µW/cm2 ( Figure 5d ). After exposure to white LED-LI and -HI, survival significantly decreased as compared with BLED and survival proportions were similar to those in pups exposed to 2×CW-LI or 3×CW-HI light.
Survival proportions after zinc deuteroporphyrin bis-glycol (ZnBG) treatment and light exposure to different fluorescent and light-emitting diode (LED) light setups. (a) Survival proportions of 3-d-old pups treated with 30 µmol ZnBG/kg and exposed to fluorescent light. Spectra of light sources taken with an Ocean Optics spectroradiometer (OOS) are shown under each respective survival plot in (b). Corresponding total irradiance measured with the OOS and the irradiance in the sensitivity range of the BiliBlanket-II (BB-II) meter from 400–520 nm (represented as the gray striped area or I400-520) are listed in Table 2 . Percentage survival and spectra of 2×cool white (CW)/1×blue fluorescent (BF), 2×BF, 2×CW, and 3×CW are displayed from left to right. After ZnBG administration mortality was 100% following exposure to 3×CW tubes at high irradiance (HI). Mortality decreased following exposure to 2×BF and 2×CW lights at low irradiance (LI). (c) Survival proportions of 3-d-old pups treated with 30 µmol ZnBG/kg and exposed to LEDs, with the corresponding spectra shown under each respective survival plot in (d). Percentage survival and spectra of blue LED (BLED), white LED (WLED)-LI, and WLED-HI are displayed from left to right. No mortality was observed after 30 µmol ZnBG/kg treatment and exposure to BLEDs. In contrast, mortality significantly increased after exposure to WLED-HI as compared with BLED (P < 0.0001) and WLED-LI exposure (P < 0.0001).
Safety and Efficacy of ZnBG
To assess the effect of light exposure on ZnBG inhibitory potency, we measured liver HO activity 3 h after administration (HO Inhibition) and light exposure (HO Inhibition+L). Up to 3.75 µmol/kg, ZnBG appeared to have no photosensitizing effects and still retained its potency, maximally inhibiting liver HO activity to 76 ± 4% ( Figure 6 ). However, inhibitory potency decreased ~17% (to 59 ± 6%) after exposure to 2×CW/1×BF.
Safety and efficacy of zinc deuteroporphyrin bis-glycol (ZnBG). Treatment of 3-d-old pups with ZnBG up to 7.5 µmol/kg appeared to have no toxic effects (white diamonds) and still maximally inhibited heme oxygenase (HO) activity to 80% (black bars). The inhibitory potency was strongly decreased following fluorescent light exposure (2×cool white/1×blue fluorescent) (white circles). The gray box indicates the estimated therapeutic window for ZnBG.
Discussion
During the past three decades, the potential use of Mps for preventing neonatal hyperbilirubinemia has been evaluated. Clinical studies of SnPP and SnMP have shown their efficacy, yet there is still no Mp available for human use. Here, we studied the photosensitizing effects of CrMP and ZnBG in 3-d-old pups. We found that CrMP shows no phototoxicity but does show chemical toxicity, with an LD50 = 23 µmol/kg. In contrast, ZnBG is phototoxic only, with an LD50 = 19.5 µmol/kg. ZnBG’s phototoxicity was dependent on dose, light source emission spectrum, and irradiance used, and was characterized by significant weight loss, decreased liver antioxidant capacity, liver injury, skin reactions, and a decreased HO inhibitory potency. However, ZnBG at doses ≤3.75 µmol/kg did not appear to be photosensitizing, but was still highly effective toward inhibiting HO.
These results confirm earlier in vitro observations describing CrMP as not photoreactive (20). Although we found no photosensitizing effects of CrMP in vivo, its safe use in neonates is concerning because of its chemical toxicity, which was previously shown in rabbits (23). Because of its high HO inhibitory potency (24), CrMP’s toxicity may occur only beyond therapeutic doses. However, we still found 30% inhibition of liver HO activity 5 wk after a single intraperitoneal dose of 7.5 µmol/kg (data not shown), suggesting a long duration of action and poor elimination. Furthermore, accumulation in tissues to toxic levels after multiple applications is conceivable. Scheingraber et al. (25) demonstrated that 40 µmol CrMP/kg intravenously decreases mean arterial pressure, sinusoidal diameter, and blood flow in rats. Moreover, it induced hemolysis, marked inflammatory responses, and AST levels. It is also possible that the trivalent chromium (Cr3+), which alters liver structures (26), is released from the porphyrin ring. Whether these effects of CrMP and/or Cr3+ are responsible for its toxicity observed in this study is uncertain.
As compared with SnPP (LD50 = 11.7 µmol/kg), ZnBG is less phototoxic, but has a higher toxicity than SnMP (42% mortality at 30 µmol/kg) (18). Our determined LD50 for ZnBG+L is consistent with earlier reports in 1-d-old Wistar rats (LD50 = 23 µmol/kg) (27). Although species, light exposure time, and experimental setup were different, the LD50s are relatively similar. In general, Zn porphyrins are weak photosensitizers as compared with Sn analogs (28,29), leading us to assume that the higher photosensitivity of ZnBG as compared with SnMP could be attributed to its BG structure. As compared with deuteroporphyrins with various central metal atoms, deuteroporphyrin BG derivatives (except FeBG) showed significant greater photoreactivity in vitro (30). In aqueous solutions, ZnBG is expected to be mainly monomeric because of its more hydrophilic side chains. Some speculate that the aggregation of porphyrins is a major factor determining the quantum yield of singlet oxygen production and that monomeric porphyrins produce singlet oxygen in a greater yield than those aggregated (31).
One striking finding of our study was the absence of ZnBG phototoxicity after BLED exposure. Delaney et al. (32) demonstrated that the triplet lifetime of SnPP decreases ~95% when excited at 450 nm. Like SnPP, ZnBG shows similar absorptions peaks at 407, 538, and 575 nm. The narrow BLED emission spectrum (spectral width: 425–525 nm, Table 2 , Figure 5d ) may just minimally react with the absorption peaks of ZnBG and therefore does not excite the molecule to a significant degree. In contrast, BF light ( Table 2 , Figure 5b ) has a wider emission spectrum (spectral width: 400–560 nm), including several mercury lines at 403, 435, 545, and 575 nm, which may be responsible, in addition to the higher total irradiance measured with the Ocean Optics spectroradiometer (OOS), for the significant higher mortality in pups exposed to BF light. An increase in irradiance appeared to directly correlate with higher mortality. Regarding the spectral qualities of the light source, full-spectrum visible light, independent of the source, appeared to be the most harmful to ZnBG-treated pups. White LED spectrum shows two distinct emission ranges, 405–475 and 475–740 nm ( Figure 5D ). Because the shorter wavelength emission range is equal to BLEDs, the higher toxicity may be because of the longer wavelength emission. Further studies are needed to determine if the ZnBG absorption peaks at 538 and 575 nm may have a higher impact on the photoreactivity than the absorption at the Soret peak or if the harm of longer wavelengths is because of a deeper penetration into the skin.
We also found a significant increase in LP in livers after 30 µmol ZnBG/kg+L. There may be a causal association between increased peroxidation with elevated ASTs and the weight loss of ZnBG+L-treated pups. Other studies documented a positive correlation between LP and increased ASTs (33,34). Weight loss may be due to LP tissue damage, leading to alterations of membrane integrity. Also, skin and respiratory water loss as underlying mechanisms have been shown to play a role (18). The significant decreases in ALP levels for the ZnBG+L group were unexpected. Simionatto et al. (35) reported that SnPP has no effect on bile flow in bile duct-cannulated rats. Therefore, we assume that ZnBG did not decrease ALP through an increased excretion into the bile. ALP is a zinc-dependent metallo-enzyme, and there is evidence that high plasma Zn2+ ion levels can depress ALP activity (36). However, whether light exposure and subsequent photobleaching of ZnBG lead to a release of Zn2+ from the porphyrin ring is speculative.
Confirming that phototherapy can decrease antioxidant capacity, we found elevated liver LP and elevated AST activities in VEH+L pups, although these were not statistically significant. In contrast, ZnBG-treated pups had LP and AST levels equal to those of VEH pups. Histological examination of liver sections taken from ZnBG+L treated pups, 24 h and 6d after treatment, showed no morphological changes as compared with livers of VEH pups (data not shown). These findings suggest that the use of ZnBG in a safe treatment strategy, such as protection from intense light exposure, appears less harmful than phototherapy itself. We also performed a pilot experiment treating 3-d-old pups (n = 6) with 30 µmol ZnBG/kg and initiating light exposure 3 h post-ZnBG treatment. No mortality was observed (data not shown). Moreover, antioxidant capacity and AST levels of 3.75 µmol/kg ZnBG+L did not differ from VEH+L treatment. Injection of 10 mg Vit E/kg intramuscularly 24 h before 30 µmol/kg ZnBG+L protected livers against LP. Although no reduction in mortality was seen (data not shown), the use of antioxidants could be protective, at least in part, against ZnBG+L-mediated tissue damage. This, in particular, is important in a human neonates, because β-carotene, gluthathione, Vit E, and catalase are at very low levels and therefore the antioxidant capacity of tissues is limited (37).
The results of this study characterize CrMP as a compound with no phototoxicity, but one with chemical toxicity. Although its LD50 may be beyond therapeutic doses, its potential clinical use is concerning because of its long duration and risk of accumulation in target tissues. In contrast, ZnBG is photosensitizing but appears to be safe under light-restricted conditions or in low doses. We observed no photosensitizing effects at 3.75 µmol ZnBG/kg+L. The ZnBG-mediated phototoxicity was dependent primarily on its dose but also on the light source emission spectrum and irradiance. Considering its high inhibitory potency and short duration of action, low doses of ZnBG may be a safe therapeutic option in the treatment of neonatal hyperbilirubinemia.
Materials and Methods
Reagents
Mps. ZnBG (5.6 mg) and CrMP (5.2 mg) (Frontier Scientific, Logan, UT) were prepared as previously described (19,21) to yield 4 mM stock solutions. VEH was prepared similarly without the addition of Mps. All solutions were prepared in subdued light and stored at 4 °C in the dark until use.
Methemalbumin. Hemin and bovine serum albumin (BSA) (Sigma-Aldrich, St Louis, MO) were dissolved as previously described (19,21) to yield a stock solution of methemalbumin (1.5 mM heme/0.15 mM BSA).
Nicotinamide adenine dinucleotide phosphate-oxidase. A 4.5 mM solution was made by dissolving 3.8 mg nicotinamide adenine dinucleotide phosphate, reduced (Calbiochem, San Diego, CA) in 1ml buffer (0.1M K2HPO4/KH2PO4, pH 7.4).
Butylated hydroxytoluene. Butylated hydroxytoluene (10 mM) was dissolved in 95% ethanol.
Vit E. Vit E (50 mg/ml, VIPRIMOL, Hofmann-La Roche, Nutley, NJ) was diluted to 1 mg/ml with saline. A total of 10 µl was injected intramuscularly to reach a final dose of 10 mg/kg.
Materials
For most experiments, two CW (F20T12CW; Sylvania, Danvers, MA,) flanking one BF tube (TL20W/52; Philips, Andover, MA) were placed overhead 20 ± 1.0 cm from the pups, which delivered a spectral irradiance of 35.0 ± 1.0 µW/cm2/nm. In addition, other exposure setups were used (see Table 2 ). All fluorescent tubes were fitted with reflectors to improve light distribution. Unless indicated differently, irradiance was measured using a BiliBlanket Meter II (BB-II, Ohmeda, GE Healthcare, Fairfield, CT), which measures irradiance in the range of 400–520 nm (peak sensitivity = 450 ± 10 nm, bandwidth = 60 nm). The BB-II is a commonly used meter and its manufacturer claims that it can measure irradiance of fluorescent, halogen, and fiberoptic light sources (15,38). Lamp emission spectra and irradiance was also measured using a flat-response fiberoptic spectrometer (OOS, Ocean Optics S2000, Dunedin, FL), having a measurement range of 200–1100 nm. For each light source, light emission distance to the pup was adjusted to achieve a total irradiance of 2,115 ± 200 µW/cm2, defined as LI, and 5,175 ± 125 µW/cm2, defined as HI.
Animals
FVB breeders were obtained from Charles River Laboratories (Wilmington, MA). Post-partum, pups were kept with the dam and maintained in the Department of Comparative Medicine (Stanford University, Stanford, CA) until 3-d-old. We used 3-d-old pups because they have no fur (hair) and very thin skin, comparable with the immature translucent skin of the human preterm newborn. The study was approved by Stanford University’s Institutional Animal Care and Use Committee.
Experimental Protocol
Pups were randomly given VEH, CrMP, or ZnBG (3.75–30.0 µmol/kg) intraperitoneally, immediately placed in an open plastic tray (8 × 11.5 × 2.5 cm) lined with wet paper towels, and exposed to overhead light for 3 h (VEH+L; CrMP+L; and ZnBG+L). The temperature measured at pup level was 27 ± 1.0 °C. Pups were misted with water every 30 min for hydration. Age-matched pups, with and without ZnBG or CrMP, were returned to mothers and kept under subdued light (VEH; CrMP; and ZnBG). Unless otherwise indicated, all experiments were performed using the 2×CW/1×BF light setup.
Histological Staining
At 6 d post-light exposure, skin samples were harvested and placed in 10% formalin, embedded in paraffin after 24 h according to standard protocols, and sectioned (6 µm) using a microtome. Deparaffinized sections were stained with hematoxylin and eosin (American Master*Tech Scientific, Lodi, CA).
Serum Biochemistry
Immediately post-light exposure, pups and controls were killed and blood was collected in lithium-heparin gel tubes (CapiJect, Terumo Medical, Somerset, NJ) and centrifuged at 13,000 g × 1 min to separate red blood cells. Blood from three pups was pooled for measurements of AST and ALP levels (Diagnostic Laboratories, Department of Comparative Medicine, Stanford, CA).
Tissue Preparation
After animals were killed, harvested tissues were immediately rinsed with ice-cold buffer, prepared as previously described (19,21), and kept on ice until HO activity or LP was measured.
Lipid Peroxidation
LP measurements were performed as previously described (22). The standard reaction mixture, containing 6 µM FeSO4 (Fe2+), 100 µM potassium ascorbate in buffer (80 µl), tissue sonicate (20 µl = 2 mg FW), and 1 µl butylated hydroxytoluene (100 µM, n = 2 blank reactions) was pipetted into n = 5 amber glass vials. Capped vials were purged with CO-free air and incubated at 37 °C for 30 min. Reactions were terminated by adding 10 µl sulfosalicylic acid (15%, w/v) and placing vials in wet ice. CO released into the headspace was measured by gas chromatography, calculated as pmol CO/h/mg FW, and expressed as fold change from VEH.
HO Activity
A total of 20 µl sonicate was incubated for 15 min at 37 °C in CO-free septum-sealed glass vials containing 20 µl 150 µM/15 µM methemalbumin and 20 µl 4.5 mM β nicotinamide adenine dinucleotide phosphate, reduced as previously described (39). Blank reaction vials contained buffer instead of β nicotinamide adenine dinucleotide phosphate, reduced. Reactions were terminated by adding 5 µl sulfosalicylic acid and placing the vials in wet ice. CO released into the headspace was quantitated by gas chromatography. HO activity was calculated as pmol CO/h/mg FW and expressed as % inhibition of HO activity.
Data Analyses
Data are presented as mean ± SD. Kaplan–Meier plots were used to determine survival over time, and compared using the log-rank test. One-way ANOVA, followed by Bonferroni’s multiple comparison test, was used to compare groups. Differences were considered significant when P ≤ 0.05.
Statement of Financial Support
This work was supported by National Institutes of Health grant 1HD058663-01A2, the Mary L. Johnson Research Fund, the Christopher Hess Research Fund, and the H.M. Lui Research Fund.
References
American Academy of Pediatrics. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297–316.
Morris BH, Oh W, Tyson JE, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med 2008;359:1885–96.
Hintz SR, Stevenson DK, Yao Q, et al. Is phototherapy exposure associated with better or worse outcomes in 501- to 1000-g-birth-weight infants? Acta Paediatr 2011;100:960–5.
Kurt A, Aygun AD, Kurt AN, Godekmerdan A, Akarsu S, Yilmaz E . Use of phototherapy for neonatal hyperbilirubinemia affects cytokine production and lymphocyte subsets. Neonatology 2009;95:262–6.
Drummond GS, Kappas A . Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci USA 1981;78:6466–70.
Maines MD . Zinc. protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim Biophys Acta 1981;673:339–50.
Tenhunen R, Marver HS, Schmid R . The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 1968;61:748–55.
Balla G, Jacob HS, Balla J, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 1992;267:18148–53.
Tenhunen R, Ross ME, Marver HS, Schmid R . Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry 1970;9:298–303.
Appleton SD, Chretien ML, McLaughlin BE, et al. Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab Dispos 1999;27:1214–9.
Luo D, Vincent SR . Metalloporphyrins inhibit nitric oxide-dependent cGMP formation in vivo. Eur J Pharmacol 1994;267:263–7.
Zhang W, Contag PR, Hardy J, et al. Selection of potential therapeutics based on in vivo spatiotemporal transcription patterns of heme oxygenase-1. J Mol Med 2002;80:655–64.
Fort FL, Gold J . Phototoxicity of tin protoporphyrin, tin mesoporphyrin, and tin diiododeuteroporphyrin under neonatal phototherapy conditions. Pediatrics 1989;84:1031–7.
McDonagh AF, Palma LA . Tin-protoporphyrin: a potent photosensitizer of bilirubin destruction. Photochem Photobiol 1985;42:261–4.
Vreman HJ, Gillman MJ, Downum KR, Stevenson DK . In vitro generation of carbon monoxide from organic molecules and synthetic metalloporphyrins mediated by light. Dev Pharmacol Ther 1990;15:112–24.
Kappas A, Drummond GS, Manola T, Petmezaki S, Valaes T . Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics 1988;81:485–97.
Valaes T, Petmezaki S, Henschke C, Drummond GS, Kappas A . Control of jaundice in preterm newborns by an inhibitor of bilirubin production: studies with tin-mesoporphyrin. Pediatrics 1994;93:1–11.
Hintz SR, Vreman HJ, Stevenson DK . Mortality of metalloporphyrin-treated neonatal rats after light exposure. Dev Pharmacol Ther 1990;14:187–92.
Morioka I, Wong RJ, Abate A, Vreman HJ, Contag CH, Stevenson DK . Systemic effects of orally-administered zinc and tin (IV) metalloporphyrins on heme oxygenase expression in mice. Pediatr Res 2006;59:667–72.
Vreman HJ, Ekstrand BC, Stevenson DK . Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr Res 1993;33:195–200.
He CX, Campbell CM, Zhao H, et al. Effects of zinc deuteroporphyrin bis glycol on newborn mice after heme loading. Pediatr Res 2011;70:467–72.
Vreman HJ, Wong RJ, Sanesi CA, Dennery PA, Stevenson DK . Simultaneous production of carbon monoxide and thiobarbituric acid reactive substances in rat tissue preparations by an iron-ascorbate system. Can J Physiol Pharmacol 1998;76:1057–65.
Lutton JD, Abraham NG, Drummond GS, Levere RD, Kappas A . Zinc porphyrins: potent inhibitors of hematopoieses in animal and human bone marrow. Proc Natl Acad Sci USA 1997;94:1432–6.
Wong RJ, Vreman HJ, Schulz S, Kalish FS, Pierce NW, Stevenson DK . In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J Perinatol 2011;31:Suppl 1:S35–41.
Scheingraber S, Messner S, Matt S, et al. Metalloporphyrins, used for HO-1 inhibition, themselves affect hepatic microcirculation, liver function, and hepatocellular integrity. Microcirculation 2009;16:355–63.
Silva RF, Lopes RA, Sala MA, et al. Action of trivalent chromium on rat liver structure. Histometric and haematological studies. Int J Morphol 2006;24:197–203.
Vreman HJ, Lee OK, Stevenson DK . In vitro and in vivo characteristics of a heme oxygenase inhibitor: ZnBG. Am J Med Sci 1991;302:335–41.
Scott J, Quirke JM, Vreman HJ, Stevenson DK, Downum KR . Metalloporphyrin phototoxicity. J Photochem Photobiol B, Biol 1990;7:149–57.
Vreman HJ, Stevenson DK . Metalloporphyrin-enhanced photodegradation of bilirubin in vitro. Am J Dis Child 1990;144:590–4.
Vreman HJ, Cipkala DA, Stevenson DK . Characterization of porphyrin heme oxygenase inhibitors. Can J Physiol Pharmacol 1996;74:278–85.
Valenzeno DP . Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem Photobiol 1987;46:147–60.
Delaney JK, Mauzerall D, Drummond GS, Kappas A . Photophysical properties of Sn-porphyrins: potential clinical implications. Pediatrics 1988;81:498–504.
Cerný D, Lekic N, Vánová K, et al. Hepatoprotective effect of curcumin in lipopolysaccharide/-galactosamine model of liver injury in rats: relationship to HO-1/CO antioxidant system. Fitoterapia 2011;82:786–91.
Kain V, Kumar S, Puranik AS, Sitasawad SL . Azelnidipine protects myocardium in hyperglycemia-induced cardiac damage. Cardiovasc Diabetol 2010;9:82.
Simionatto CS, Anderson KE, Drummond GS, Kappas A . Studies on the mechanism of Sn-protoporphyrin suppression of hyperbilirubinemia. Inhibition of heme oxidation and bilirubin production. J Clin Invest 1985;75:513–21.
Fishman WH, Wayne A, Homburger F . The evaluation of diagnostic tests for cancer; inhibition of serum alkaline phosphate by zinc ion; the Roche test. Cancer Res 1949;9:681–3.
Dennery PA, Vreman HJ, Rodgers PA, Stevenson DK . Role of lipid peroxidation in metalloporphyrin-mediated phototoxic reactions in neonatal rats. Pediatr Res 1993;33:87–91.
Vreman HJ, Wong RJ, Murdock JR, Stevenson DK . Standardized bench method for evaluating the efficacy of phototherapy devices. Acta Paediatr 2008;97:308–16.
Vreman HJ, Stevenson DK . Detection of heme oxygenase activity by measurement of CO. Unit 9.2. In: Maines MD, Costa G, Reed DJ, Sassa S, Sipes IG, eds. Current Protocols in Toxicology. New York: Wiley; 1999:9.2.1–9.2.10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulz, S., Wong, R., Kalish, F. et al. Effect of light exposure on metalloporphyrin-treated newborn mice. Pediatr Res 72, 161–168 (2012). https://doi.org/10.1038/pr.2012.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.62