Abstract
Conflicting evidence exists on the effect of long-chain polyunsaturated fatty acid (LCPUFA) formula supplementation on cardiovascular health in term infants. It is known that LCPUFA supplementation does not affect infant growth, but long term outcome data are not available. The current study investigates whether 2 mo LCPUFA formula supplementation affects cardiovascular and anthropometric development at 9 y. A prospective, double-blind, randomized trial was performed in healthy term infants: a standard formula control group (CF, n = 169) and a LCPUFA-supplemented group [LF, n = 145; 0.45% (by wt) AA and 0.30% (by wt) docosahexaenoic acid (DHA)]. A breastfed group (BF; n = 159) served as reference. At the age of 9 y, systolic and diastolic blood pressure, heart rate, head circumference, weight, and height were measured. Univariate and multivariate analyses were performed; 63 to 79% of children were assessed. None of the cardiovascular or anthropometric measurements differed between the formula groups. Breastfed children had a marginally lower heart rate than formula-fed children, in particular compared with children fed control formula. Blood pressure and parameters of growth including BMI of breast and formula-fed children did not differ. In conclusion, the study suggests that short-term LCPUFA supplementation does not influence cardiovascular and anthropometric development at 9 y.
Similar content being viewed by others
Main
The notion that breast feeding has consistently been associated with a modest beneficial effect on blood pressure in later life is relevant for public health (1,2). The underlying mechanisms of the association are not clear. Long-chain polyunsaturated fatty acids (LCPUFAs) may be candidates.
Until recently, LCPUFAs were not present in standard formula. Formula only contained the precursor essential fatty acids, alpha-linolenic acid (ALA,18:3n-3), and linoleic acid (LA, 18:2n-6). As young infants have a limited capacity to synthesize docosahexaenoic acid (DHA) and arachidonic acid (AA) from these precursors (3), LCPUFA levels in plasma or red blood cell membrane gradually decline in infants fed nonsupplemented formulae indicating a relative LCPUFA deficiency. Based on the advantageous effect of LCPUFA supplementation on neurodevelopment assessed at young age, it is advised that infant formulae should contain LCPUFA in quantities comparable with breast milk (4). Consequently, several of the formulas currently on the market contain LCPUFAs. However, there is still an ongoing debate concerning the long-term consequences of dietary LCPUFAs, including the effect on blood pressure regulation.
Studies on the effects of early LCPUFA supplementation on cardiovascular indices in humans, such as blood pressure and heart rate, are limited in number and heterogeneous in methodological quality (Table 1). They also differ in the duration of supplementation, fatty acid dosage, and lipid composition. Two studies on early postnatal supplementation with LCPUFAs (5,6) and one in late infancy (7) reported beneficial effects of LCPUFAs on blood pressure and heart rate in later life. Two other studies however found no effect of LCPUFA supplementation in early life (8,9) and one study even found a negative effect in school age boys (10).
Two recent meta-analyses indicated that early postnatal LCPUFA supplementation in term infants does not affect growth measures such as head circumference, weight, length, and the resulting BMI until the age of 18 mo (11,12). Breastfeeding, on the other hand, is associated with a small but consistent reduction in obesity risk in later childhood (13). However, it is debated whether the effect is mediated by the nutritional contents of human milk or by associated factors, such as less maternal smoking during pregnancy and a maternal BMI <25 (Ref. 14).
The current study is part of the Groningen LCPUFA study, a randomized controlled trial on the effect of supplementation of formula with LCPUFAs during the first two postnatal months in healthy term infants. The focus of the study is neurodevelopmental outcome, but also some other indicators of child health are evaluated (15). Previously, the data of our study groups were used in a meta-analysis, which indicated that LCPUFA supplementation and breastfeeding were not associated with growth advantages at 18 mo (12). Recently, the participants of the study were reassessed at 9 y. Here, we report on cardiovascular health expressed as blood pressure and heart rate and anthropometrics in terms of weight, length, BMI, and head circumference.
The primary aim of the current article is to explore whether the short-term postnatal LCPUFA intervention was associated with blood pressure, heart rate, and anthropometric development at 9 y. In addition, we explored whether breast feeding was associated with a beneficial effect on blood pressure, heart rate, and anthropometric development.
METHODS
Subjects.
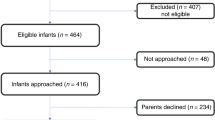
The study is part of a double-blind randomized controlled trial investigating the effect of LCPUFA supplementation on the development of healthy term infants (the Groningen LCPUFA study). Details on the study design, including exact diet composition, have been described previously (15). Between 1997 and 1999, expecting mothers were recruited during pregnancy checkup visits at the University and Martini Hospitals in Groningen and at midwife clinics in and near Groningen. Final enrollment occurred in the neonatal period. All infants were born at 37–42 wk of gestation. Mothers of 314 infants chose to bottle feed their child and 160 opted for breastfeeding. The infants receiving formula were randomized into a standard formula group [control formula (CF; n = 169)] and a LCPUFA-supplemented formula group (LF, n = 145). Standard formula consisted of Nutrilon Premium. For the supplemented formula, the lipid fraction of Nutrilon Premium was enriched with 0.45% (by wt) AA and 0.30% (by wt) DHA. DHA was derived from egg yolk and tuna oil that was low in eicosapentaenoic acid, and the source of AA was egg yolk and a single cell oil produced by a common soil fungus, Mortierella alpina. The LCPUFAs were provided as mix of phospholipids (15%) and triglycerides (85%) to mimic the composition of breast milk. All infants received their assigned diets beginning at age 1–5 d. Supplementation lasted till the end of the second postnatal months. In case breastfeeding stopped before 2 mo, the infant received LCPUFA-supplemented formula till the full age of 2 mo. All formula-fed infants received CF from two completed months until the age of 6 mo. All 436 children (92% of the original groups) assessed at 18 mo were eligible for re-examination at 9 y. At the 9 y follow-up, both parents and examiners were unaware of the type of formula feeding the infant had received. The examiners were also blind to formula versus breast status.
Protocol and measurements.
In the current follow-up, the children and their parents were contacted, and depending on the wish of the participants, assessments were carried out either at the hospital or at home. Three hundred forty-one children agreed to participate: 91 in the LF group (63%), 123 in the CF group (73%), and 127 in the breastfed group (BF group; 79%; Fig. 1). During the study, type of feeding was assessed prospectively by daily diaries filled in by the mothers until the infant was 8 mo. Duration of breastfeeding varied from 1 to 56 wk; 50% of mothers exclusively breastfed beyond 2 mo. The assessments of cardiovascular parameters and anthropometrics were part of a more extensive evaluation focusing on neurodevelopmental outcome, see Ref. 16.
Blood pressure and heart rate were measured immediately after a 15-min rest period, measurement took place on the left arm with the child sitting on a chair and the arm resting on a table. An automated blood pressure monitor was used (Datascope Accutorr plus; Datascope corporation, Mahwah) with a small adult cuff (10.6–23.9 cm size bladder; measuring with a precision of micrometers Hg). This procedure was performed twice during the assessment with an interval of an hour. In all children, heart rate and systolic and diastolic blood pressure were recorded as the mean of the two readings.
Weight was measured using a calibrated Radwag scale (Radwag; Radom, Poland; which measured with a precision of 500 mg) and body length using a Seca stabilometer (Seca Deutschland, Hamburg, Germany; which measured with a precision of millimeters). BMI was determined using international standards, taking gender and age into account (17). Occipitofrontal head circumference was assessed using a nonstretchable “lasso” tape with a precision of millimeters.
Data on pre- and perinatal conditions had been collected during enrollment with the help of the obstetric optimality score (OOS).The OOS describes the obstetric conditions ranging from the parents' socioeconomic status to the infant's condition immediately after birth (18). Anthropometrics had been recorded at birth and at 3 and 18 mo (12). At the 9-y follow-up, information was collected on parental education and profession, the child's medical history, nutritional habits, and family composition (Table 2).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Groningen University Hospital. Written informed consent was obtained from all subjects. The trial is registered under ISRCTN52788665.
Data analysis.
The study was originally designed to detect a difference of 7.5 on the mental developmental index of the Bayley test of infant development at the age of 18 mo between the LF and CF groups (assuming SD = 15, power 80% at 5% significance level). Accordingly, at least 57 children had to be included in each formula group.
Statistical analysis focused on differences between the two randomized groups; in addition, differences between the formula groups and the BF group were analyzed. The anthropometric and blood pressure data, apart from the children's BMI, were normally distributed. We used independent samples t tests for crude comparison of the feeding groups and linear regression analysis for comparison adjusted for potential confounders. The children's BMI was dichotomized into normal (≤25)/overweight (>25), χ2 tests were used for crude comparisons between the feeding groups and logistic regression analyses for comparisons adjusted for potential confounders.
Factors included into the multivariate analyses were variables associated with outcome at p < 0.20: gender, maternal level of education (1 = low, 2 = medium, and 3 = high), smoking during pregnancy (yes or no), duration of the second stage of delivery, Apgar score at 1 and 3 min, birthweight, OOS, and prepregnancy maternal BMI. In line with other developmental studies, smoking was dichotomized into no/minimal smoking defined as <5 cigarettes per day and evident smoking defined as ≥5 cigarettes per day (19). Specific attention was paid to duration of exclusive breastfeeding as potential confounder. Anthropometric parameters at 3 and 18 mo were not included in the multivariate analyses as they were not regarded as confounders but as outcome variables.
Relative risk analyses were used to compare risk between the two formula groups and between the formula and BF groups for having a high blood pressure (diastolic or systolic blood pressure at or above the 90th percentile) and for being overweight (BMI >25). A p value of 0.05 or less was considered as statistically significant. Statistical analyses were performed using SPSS 14.0 for Windows (SPSS, Inc., Chicago, IL).
RESULTS
In general, obstetric and social characteristics of children assessed at 9 y were comparable with those not participating in the follow-up at 9 y (data not shown). Nonetheless some selective attrition was present, the children who took part in the follow-up showed a less optimal neuromotor condition at 3 mo (p = 0.003). Moreover, part of the attrition was group specific, more boys in the LF group did not partake (35 boys and 17 girls) compared with both the BF group (15 boys and 17 girls) and the CF group (24 boys and 17 girls). In addition, in the LF group, more children with a lower mental development index at 18 mo were lost to attrition (p = 0.007; see Ref. 16). Attrition was, however, not selective for parameters of growth at birth and during infancy (data not shown).
Cardiovascular and anthropometric outcomes in the LF and CF groups were similar (Tables 3 and 4). Multivariate analyses confirmed the absence of differences in systolic and diastolic blood pressure, heart rate, weight, height, BMI, and head circumference between the two formula groups. Relative risk analysis demonstrated no difference between the groups for high blood pressure [diastolic: CF, 0.875 (95%CI, 0.622–1.23); LF, 1.219 (95%CI, 0.685–2.168) and systolic: CF, 1.094 (95%CI, 0.725–1.650); LF, 0.898 (95%CI, 0.568–1.420)] nor for being overweight [CF, 0.881 (95%CI, 0.677–1.146); LF, 1.209 (95%CI, 0.787–1.856)].
The observational part of the study indicated that children of the BF group had a slightly lower heart rate than the children of the CF group (p = 0.04; effect size 0.914). Multivariate analyses confirmed the marginal difference (p = 0.049, 95%CI: −58.09 to −0.009; Table 5). Systolic and diastolic blood pressure, weight, height, BMI, and head circumference of the BF group were similar to those of the two formula groups (Tables 3 and 4). The lack of difference in these outcome parameters was confirmed in the multivariate analyses. Relative risk analysis demonstrated no difference between the groups for high blood pressure [diastolic: BF, 0.915 (95%CI, 0.602–1.389); formula, 1.058 (95%CI, 0.80–1.40) and systolic: BF, 1.104 (95%CI, 0.682–1.786); formula, 0.946 (95%CI, 0.733–1.221)] nor for being overweight [BF, 1.133 (95%CI, 0.787–1.633); formula, 0.930 (95%CI, 0.763–1.135)].
DISCUSSION
Our data indicated that LCPUFA supplementation for the duration of 2 mo in healthy term infants was not associated with a change in blood pressure, heart rate, weight, height, BMI, or head circumference nor with an enhanced risk of high blood pressure or overweight at 9 y. The study also revealed that children who were breastfed had a slightly lower heart rate at 9 y than children who had received formula.
A major limitation of the study is its attrition, which was 28%. However, considering the duration of the follow-up period, 9 y, this may be regarded as relatively favorable (20). Attrition was selective with respect to gender and cognitive development at 18 mo in the LF group suggesting that LF participants were a positive selection of the original LF group. However, attrition was not selective with respect to social class and parameters of growth at birth and during infancy (Table 3). A second limitation of the study is its relatively weak power. Group sizes had been determined on the basis of neurodevelopmental outcome at 18 mo measured with the Bayley Scales of Infant Development (21). Post hoc power analyses indicated that with α set at 0.05 current sample sizes allowed for a detection of small effect sizes (0.20) with a power of 0.80, i.e. the groups allowed for the detection of differences in blood pressure of 1.5 mm Hg, in heart rate of 2 beats per minute, in body weight of 1.5 kg, and in height of 1 cm. This means that this study was not able to detect more subtle effects on growth, blood pressure, and heart rate. It may also be regarded as a limitation that our parameters of cardiovascular health and anthropometrics were restricted to blood pressure, heart rate, bodyweight, length, BMI, and head circumference and did not encompass body composition, ECG, and blood lipid profile. A third limitation is the relatively short duration of supplementation, i.e. 2 mo. Some indications have been found that LCPUFA supplementation for 9 to 12 mo may affect visual and cognitive development (22,23), whereas supplementation for a few months does not affect developmental outcome (11). Further research is required to assess whether prolonged supplementation is associated with improved outcome, including cardiovascular and anthropometric outcome. The strengths of the study are its randomized design, the presence of information on a wide range of possibly confounding variables, and its assessor-blinded evaluation.
Previous studies on the relationship between LCPUFA supplementation and blood pressure reported inconsistent findings: LCPUFAs were associated with a positive effect on blood pressure, no effect, or even a negative effect. This heterogeneity presumably is the result of the large variation in methodological quality of the studies resulting from the use of high-risk populations, highly selective attrition, and cointervention with other nutrients. In addition, there is large variation between the studies in dosage and duration of the intervention and the ages during which the intervention was applied (Table 1). The current study concludes that LCPUFA supplementation of formula for 2 mo is not associated with blood pressure improvements of more than 1.5 mm Hg and a decrease in heart rate of more than 2 beats per minute. This also implies that smaller effect sizes or larger effects of longer periods of supplementation are not precluded. Originally, our study was one of a series in which the effect of longer periods of supplementation was also evaluated. However, our short-term supplementation study was the only one that was completed.
Breastfeeding, on the other hand, has shown a relatively consistent relation with blood pressure: it is associated with a modest decrease in blood pressure, i.e. a reduction of around 1.4 mm Hg in systolic blood pressure and 0.4 mm Hg in diastolic blood pressure (1). These breastfeeding effects are smaller than the current study was able to detect. However, we did find suggestions of a minor advantage for breastfeeding in heart rate, the other parameter of cardiovascular health. The minor reduction in heart rate may reflect a slight shift in autonomic control involving a minor decrease of sympathetic dominance. This may be associated with a minor reduction in risk for cardiovascular diseases in adulthood (24). The small size of the effect is illustrated by the fact that the advantage only reached statistical significance in the comparison with CF. Our finding of a minor beneficial effect of breastfeeding on cardiovascular indices is in line with other reports but somewhat smaller than often reported. This might be explained by the relatively short average period of breastfeeding, 53% of breastfed infants received a maximum of 8 wk exclusive breastfeeding (25), which is representative of Dutch breast feeding habits (26).
None of the anthropometric measures in this study were associated with early postnatal nutrition. The lack of effect of postnatal LCPUFA supplementation is in line with existing literature, but the lack of difference between the breastfed and the formula-fed groups is not. This might be explained by the limited ability to detect differences between the groups and to the above mentioned breastfeeding practices in the Netherlands.
To summarize, the current study suggests that LCPUFA supplementation of formula in healthy term infants for the duration of 2 mo does not influence cardiovascular and anthropometric development at the age of 9 y. Breastfeeding is found to have a marginal beneficial effect on cardiovascular development. The study underscores the need for carefully designed and well-controlled studies on the effect of LCPUFA supplementation on cardiovascular development.
Abbreviations
- AA:
-
arachidonic acid
- BF:
-
breastfed
- CF:
-
control formula
- DHA:
-
docosahexaenoic acid
- LCPUFA:
-
long-chain polyunsaturated fatty acids
- LF:
-
LCPUFA supplemented formula
- OOS:
-
obstetric optimality score
References
Martin RM, Gunnell D, Smith GD 2005 Breastfeeding in infancy and blood pressure in later life: Systematic review and meta-analysis. Am J Epidemiol 161: 15–26
Singhal A 2006 Early nutrition and long-term cardiovascular health. Nutr Rev 64: S44–S49
Larque E, Demmelmair H, Koletzko B 2002 Perinatal supply and metabolism of long-chain polyunsaturated fatty acids: importance for the early development of the nervous system. Ann N Y Acad Sci 967: 299–310
Koletzko B, Lien E, Agostoni C, Böhles HJ, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, Hoesli I, Holzgreve W, Lapillonne A, Putet G, Secher NJ, Symonds M, Szajewska H, Willatts P, Uauy R 2008 The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 36: 5–14
Forsyth JS, Willatts P, Agostoni C, Bissenden J, Casaer P, Boehm G 2003 Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ 326: 953
Pivik RT, Dykman R, Jing H, Gilchrist J, Badger T 2009 Early infant diet and the omega 3 fatty acid DHA: effects on resting cardiovascular activity and behavioral development during the first half-year of life. Dev Neuropsychol 34: 139–158
Damsgaard CT, Schack-Nielsen L, Michaelsen KF, Fruekilde M-B, Hels O, Lauritzen L 2006 Fish oil affects blood pressure and the plasma lipid profile in healthy Danish infants. J Nutr 136: 94–99
Ayer JG, Harmer JA, Xuan W, Toelle B, Webb K, Almqvist C, Marks GB, Celermajer DS 2009 Dietary supplementation with n-3 polyunsaturated fatty acids in early childhood: effects on blood pressure and arterial structure and function at age 8 y. Am J Clin Nutr 90: 438–446
Ulbak J, Lauritzen L, Hansen HS, Michaelsen KF 2004 Diet and blood pressure in 2.5-y-old Danish children. Am J Clin Nutr 79: 1095–1102
Asserhøj M, Nehammer S, Matthiessen J, Michaelsen KF, Lauritzen L 2009 Maternal fish oil supplementation during lactation may adversely affect long-term blood pressure, energy intake, and physical activity of 7-year-old boys. J Nutr 139: 298–304
Simmer K, Patole SK, Rao SC 2008 Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev 1: CD000376
Rosenfeld E, Beyerlein A, Hadders-Algra M, Kennedy K, Singhal A, Fewtrell M, Lucas A, Koletzko B, Von Kriesl R 2009 IPD meta-analysis shows no effect of LC-PUFA supplementation on infant growth at 18 months. Acta Paediatr 98: 91–97
Arenz S, Rückerl R, Koletzko B, von Kries R 2004 Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord 28: 1247–1256
Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG 2005 The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr 82: 1298–1307
Bouwstra H, Dijck-Brouwer DA, Wildeman JA, Tjoonk H, van der Heide J, Boersma E, Muskiet FA, Hadders-Algra M 2003 Long-chain polyunsaturated fatty acids have a positive effect on the quality of general movements of healthy term infants. Am J Clin Nutr 78: 313–318
de Jong C, Kikkert HK, Fidler V, Hadders-Algra M 2010 The Groningen LCPUFA study: no effect of postnatal long-chain polyunsaturated fatty acids in healthy term infants on neurological condition at 9 years. Br J Nutr 104: 566–572
Cole TJ 2000 Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320: 1240–1243
Touwen BC, Huisjes HJ, Jurgens-Van der Zee AD, Bierman-Van Eendenburg ME, Smrkovsky M, Olinga AA 1980 Obstetrical condition and neonatal neurological morbidity. An analysis with the help of the optimality concept. Early Hum Dev 4: 207–228
Agostoni C, Riva E, Giovannini M, Pinto F, Colombo C, Rise P, Galli C, Marangoni F 2008 Maternal smoking habits are associated with differences in infants' long-chain polyunsaturated fatty acids in whole blood: a case-control study. Arch Dis Child 93: 414–418
Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, Koletzko B, Lucas A 2008 How much loss to follow-up is acceptable in long-term randomised trials and prospective studies?. Arch Dis Child 93: 458–461
Bouwstra H, Dijck-Brouwer D, Boehm G, Boersma ER, Muskiet FA, Hadders-Algra M 2005 Long-chain polyunsaturated fatty acids and neurological developmental outcome at 18 months in healthy term infants. Acta Paediatr 94: 26–32
Drover J, Hoffman DR, Castaneda YS, Morale SE, Birch EE 2009 Three Randomized controlled trials of early long-chain polyunsaturated fatty acid supplementation on means-end problem solving in 9-month-olds. Child Dev 80: 1376–1384
Birch EE 2005 Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am J Clin Nutr 81: 871–879
Thayer JF, Lane RD 2007 The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 74: 224–242
Bouwstra H, Boersma ER, Boehm G, Dijck-Brouwer D, Muskiet FA, Hadders-Algra M 2003 Exclusive breastfeeding of healthy term infants for at least 6 weeks improves neurological condition. J Nutr 133: 4243–4245
Lanting CI, Van Wouwe JP, Reijneveld SA 2005 Infant milk feeding practices in the Netherlands and associated factors. Acta Paediatr 94: 935–942
Author information
Authors and Affiliations
Corresponding author
Additional information
Previous parts of the LCPUFA study have been funded by Numico Research B.V. The follow-up at 9 y is part of the Early Nutrition Programming Project (EARNEST), which is funded under the Food Quality and Safety Priority of the Sixth Framework Programme for Research and Technical Development of the European Community (FOOD-CT-2005-007036, www.metabolic-programming.org).
Rights and permissions
About this article
Cite this article
De Jong, C., Boehm, G., Kikkert, H. et al. The Groningen LCPUFA Study: No Effect of Short-Term Postnatal Long-Chain Polyunsaturated Fatty Acids in Healthy Term Infants on Cardiovascular and Anthropometric Development at 9 Years. Pediatr Res 70, 411–416 (2011). https://doi.org/10.1203/PDR.0b013e31822a5ee0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31822a5ee0
This article is cited by
-
Breast milk n-3 long-chain polyunsaturated fatty acids and blood pressure: an individual participant meta-analysis
European Journal of Nutrition (2021)