Abstract
The first months of life represent a critical period for the maturation of the infant's immune system and, thus, a window of opportunity for measures to reduce the risk of disease. We hypothesized that specific probiotics might promote mucosal immunologic maturation in formula-fed infants. The numbers of cow's milk–specific and total IgA-secreting cells were measured at 3, 7, and 12 mo of age in a double-blind placebo-controlled study of 72 infants with early artificial feeding. The infants consumed infant formula supplemented with specific probiotics (Lactobacillus GG and Bifidobacterium lactis Bb-12) or placebo during the first year of life. Further analyses of the serum concentrations of the IgA-inducing cytokine TGF-β2 and the soluble innate microbial receptor sCD14 were conducted. The numbers of cow's milk–specific IgA secreting cells were significantly higher in infants receiving probiotics compared with those receiving placebo (p = 0.045, ANOVA for repeated measures). At 12 mo of age, the serum concentrations of sCD14 were 1479 pg/mL [95% confidence interval (CI) 1373–1592] in infants receiving probiotics and 1291 pg/mL (95% CI 1152–1445) in infants receiving placebo (p = 0.046). Administration of the probiotics Lactobacillus GG and Bifidobacterium lactis Bb-12 at the time of introduction of cow's milk in the infant's diet results in cow's milk–specific IgA antibody responsiveness that may be the result of increased production of sCD14.
Similar content being viewed by others
Main
The first months of life represent a critical period when the infant's immature immune system is exposed to a vast variety of microbial and dietary antigens as the intestinal microbiota is established and oral intake of new foods begins. The immunologic consequences of these early contacts appear to be decisive in the development of adequate and appropriate immune responsiveness. Cross-talk with commensal micro-organisms in the intestine results in production of immunoglobulin (Ig)A antibodies (1), which provide protection by binding and excluding microbial and dietary antigens on the mucosal surfaces. Transforming growth factor (TGF)-β is the initial trigger for IgA production (2) and, indeed, expression of TGF-β is directly correlated with the mucosal IgA antibody response (3).
Infants with limited exclusive breast-feeding appear to exhibit heightened morbidity due to increased risk of infectious (4,5) and atopic disease (6). This may be explained by the fact that breast milk contains several factors, including nutrients, antimicrobial agents, IgA antibodies, TGF-β, and the innate microbe receptor soluble (s)CD14, which may contribute beneficially to the immunologic maturation and well-being of the infant (7–9). In addition, breast milk contains viable lactobacilli (10) and factors that promote the growth of bifidobacteria in the infant's intestine (11,12). These microbes may modulate host immune responses by processing luminal antigens and by directly stimulating the mucosal immune system. We therefore hypothesized that specific probiotics, providing a microbial stimulus, might promote the mucosal immunologic maturation in formula-fed infants. To test this hypothesis, we assessed the development of IgA-secreting cells and serum concentrations of sCD14 and TGF-β2 during the first year of life in a randomized, double-blind, placebo-controlled study of infants who required infant formula before the age of 2 mo.
METHODS
Subjects and study design.
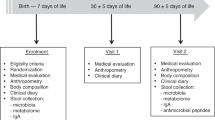
The present investigations were conducted as a part of a double-blind, placebo-controlled trial evaluating the efficacy and safety of infant formula supplemented with probiotics in promoting infant health. The sole inclusion criterion for the study was the need for artificial feeding before the age of 2 mo. Infants with chronic disease were excluded from the study.
The trial was primarily designed to assess the effects of probiotic supplementation in reducing the risk of infectious disease in infants devoid of the recommended 6 mo of exclusive breast-feeding. The limitations of the study include firstly that no conclusive data were available at recruitment concerning the primary endpoints in infants who were not exclusively breast-fed, upon which reliable power calculations could be based. Secondly, due to the baby-friendly hospital initiative and the strong policy of promoting breast-feeding, which is of utmost importance in well-baby clinics, advertising the study with formula supplementation at an early age was not considered acceptable by the authors. Consequently, information regarding the study was given only when the need of formula was imminent. All families who contacted the research personnel during the recruitment period between September 2000 and May 2002 were assessed for eligibility. One infant with cleft palate was excluded from the study.
In all, 81 infants were randomized to receive infant formula (Enfamil, Mead Johnson Nutritionals, Evansville, IN) supplemented with either 1 × 1010 colony-forming units of both Lactobacillus rhamnosus (Lactobacillus GG, American type culture collection 53103, Valio Ltd., Helsinki, Finland) and Bifidobacterium lactis Bb-12 (Chr. Hansen, Hoersholm, Denmark) or placebo (microcrystalline cellulose) daily until the age of 12 mo. The intervention was commenced when the need for artificial feeding arose and the supplemented formula was to be used as the sole infant formula during the study period. Originally, 38 infants were randomized to receive probiotics and 43 infants to receive placebo. The follow-up was completed by 72 of the 81 (89%) infants enrolled. Thus, altogether nine infants did not complete the follow-up: six infants receiving probiotics (of whom two discontinued the study because of gastrointestinal complaints, one because of the arduousness of the study, one family found the powdered formula inconvenient, and two infants were lost to follow-up) and three infants receiving placebo (one because of gastrointestinal complaints, one because of the arduousness of the study, and one infant was lost to follow-up). The number of infants with available blood samples for the determination of serum sCD14 and TGF-b2 concentrations was further limited as a result of difficulties concerning obtaining samples and storage. All analyses were performed according to the groups to which the infants were originally randomized. The nine infants who did not complete the follow-up were excluded from the analyses as were the infants from whom samples were not available, which may confound the effects of the original randomization.
The study was found acceptable by the Turku University Central Hospital Ethical Committee and written informed consent was obtained from the infants' parents.
Clinical examination of the infants was performed at scheduled visits at the start of intervention and subsequently at the ages of 3, 7, and 12 mo. The diagnosis of atopic eczema was made using the criteria introduced by Hanifin (13). If cow's milk allergy was suspected, the diagnosis was confirmed by a double-blind, placebo-controlled cow's milk challenge as described in detail elsewhere (14). Skin prick tests were performed at the ages of 7 and 12 mo. The tested allergens included banana, potato, carrot, apple, wheat, rice, milk, egg, cod, soybean, and gliadin. The infant was considered sensitized in case of one or more positive reaction at either time point.
Determination of the number of immunoglobulin A-secreting cells.
The total number of IgA-secreting cells and the number of cells producing IgA antibodies directed specifically against cow's milk allergens (casein and β-lactoglobulin) were measured using the ELISPOT method as described in detail elsewhere (15) from blood samples obtained at the ages of 3, 7, and 12 mo. Samples were available from 58, 54, and 63 infants at respective time points. Briefly, peripheral blood mononuclear cells were isolated using Ficoll-Paque gradient centrifugation. Isolated cells were washed three times in Hanks' balanced salt solution and suspended in RPMI 1640 medium containing 10% FCS and adjusted to a concentration of 1–2 × 106 cells/mL.
To determine the total number of IgA-secreting cells, the wells were coated for 2 h at 37°C with rabbit anti-human IgA (DAKO A/S, Glostrup, Denmark) diluted 1/100 in PBS (pH 7.4), and to detect the number of cells secreting cow's milk-specific IgA, the coating was performed with β-lactoglobulin (20 μg/mL) and casein (20 μg/mL) (both from Sigma Chemical Co., St. Louis, MO). Uncoated binding sites were blocked with 1% BSA (Roche Molecular Biochemicals, Mannheim, Germany) in PBS (pH 7.4) for 30 min at 37°C. After washings the lymphocyte suspension was incubated on antigen-coated flat-bottomed 96-well microtiter plates (Immunoplate RI, NUNC A/S, Roskilde, Denmark) at 37°C for 2 h. The detection of antibodies secreted during that time was performed with alkaline phosphatase-conjugated goat antiserum to human IgA (Sigma Chemical Co.) diluted in 1% BSA-PBS (pH 7.4) incubated overnight at room temperature, followed by a substrate agarose overlay and observation of the colored spots.
Determination of TGF-β2 and soluble CD14 in serum.
Venous blood samples were drawn, heparinized, and stored for later analysis at the ages of 3, 7, and 12 mo. Serum samples were available from 38 infants (18 infants receiving probiotics and 20 infants receiving placebo). The concentrations of TGF-β2 and sCD14 and were measured from thawed sera in duplicate using commercial sandwich ELISA specific for the molecule (R & D Systems, Minneapolis, MN). Activation of latent TGF-β2 and all determinations were made according to the manufacturer's instructions.
Statistical methods.
The baseline data are expressed as means with range. The χ2 test was used in comparisons of proportions between groups. The results are expressed as geometric means with 95% confidence intervals (CI). The effect of intervention on the number of IgA-secreting cells and serum sCD14 and TGF-β2 concentrations during the course of the follow-up were assessed after logarithmic transformation using ANOVA for repeated measures. If the ANOVA suggested a difference between groups, further analyses at each time point were conducted after logarithmic transformation using t test. A p value of <0.10 was considered statistically significant.
RESULTS
Baseline and clinical characteristics.
The baseline and clinical characteristics of the infants receiving probiotics and placebo are presented in Table 1. During the 12-mo follow-up, 4/32 (13%) of the infants receiving probiotics and 8/40 (20%) of those receiving placebo manifested with atopic eczema (p = 0.40). Cow's milk allergy was confirmed in 0/32 (0%) of the infants receiving probiotics and 3/40 (8%) infants receiving placebo (p = 0.11). The respective proportions of infants with evidence of sensitization were 2/32 (6%) and 3/40 (8%) (p = 0.84). None of the infants receiving probiotics and one infant (3%) receiving placebo was sensitized to cow's milk (p = 0.37).
Total and cow's milk-specific IgA-secreting cells.
The total numbers of IgA-secreting cells at 3, 7, and 12 mo of age were not statistically significantly different between infants receiving probiotics and placebo as assessed by ANOVA for repeated measures (p = 0.29) (Fig. 1). The respective numbers of cow's milk–specific IgA-secreting cells during the same time are presented in Figure 2 (p = 0.045, ANOVA for repeated measures).
The numbers of cow's milk-specific IgA-secreting cells (per 106 cells) in infants receiving probiotics (white bars) and placebo (gray bars) at 3, 7, and 12 mo of age. (The bars represent geometric means and the whiskers represent the 95% CI; the p values correspond to t test after logarithmic transformation.)
Concentrations of TGF-β2 and sCD14 in serum.
The differences in the serum concentrations of TGF-β2 at 3, 7, and 12 mo of age (Fig. 3) were not statistically significant between infants receiving probiotics and placebo as assessed by ANOVA for repeated measures (p = 0.23).
The serum concentrations of sCD14 in infants receiving probiotics and placebo at 3, 7, and 12 mo of age are presented in Figure 4 (p = 0.06, ANOVA for repeated measures). At the end of the follow-up, the serum concentrations of sCD14 were 1479 pg/mL (95% CI 1373–1592) and 1291 pg/mL (95% CI 1152–1445) in infants receiving probiotics and placebo, respectively (p = 0.046).
DISCUSSION
We found that probiotic supplementation commenced at the time of introduction of cow's milk in the diet was associated with a significant increase in the number of cow's milk-specific IgA-secreting cells during the first months of life, whereas no significant differences between the groups were found in the total number of IgA-secreting cells. Previously, probiotics have been reported to augment antigen-specific IgA responses during rotavirus infection (16) and after polio vaccination (17). According to a recent report (1), interaction with indigenous intestinal microbes induces protective mucosal IgA responses in a murine model, which was interpreted to ensure tolerance to commensal bacteria. The ELISPOT method applied in this study reliably reflects mucosal immune responsiveness (15,18) and thus our results may extend this notion of the role of intestinal microbial stimulation to establishing tolerance to dietary antigens.
In previous studies, probiotics have been shown to reduce the risk of atopic eczema but no effects on atopic sensitization have been reported (19,20).
In accordance with this, probiotic supplementation did not appear to have an effect on the risk of sensitization to cow's milk or other dietary antigens in the present study. The incidence of atopic eczema did not differ significantly between the groups. Interestingly, none of the infants receiving probiotics developed cow's milk allergy whereas 8% of the infants receiving placebo did. The difference was not statistically significant, however, and it must be emphasized that the study was not designed to primarily assess the effects of probiotics on the incidence of atopic or allergic disease.
We suggest that the observed increased IgA production achieved with probiotics in this and previous studies may in part be mediated via augmented antigen-specific TGF-β responses, as the expression of TGF-β correlates with the mucosal IgA antibody response (3). Moreover, intestinally induced antigen-specific TGF-β-secreting T cells are distributed systemically and produce TGF-β upon reactivation (21). We have previously demonstrated that administration of Lactobacillus GG increases mucosal TGF-β2 concentrations (22) but the present study failed to demonstrate a probiotic effect on serum TGF-β2 concentrations. Nonetheless, serum cytokine concentrations display wide variability and reflect mucosal immune responses inadequately.
Recently, our understanding of the complex interactions between commensal, environmental, and pathogenic microbes and the host has significantly improved. The requirement of innate immune responses to enteric bacteria in the maintenance of a disease-free state is well established in experimental animal models (23,24). The role of innate immunity in recognition of microbes and directing immune responses in host-commensal crosstalk has gained increasing attention with the discovery of microbe recognition molecules such as Toll-like receptors (TLR) and CD14. CD14 is a co-receptor for TLR, which recognizes structures from both Gram-negative and Gram-positive bacteria (25,26) and is found in its soluble form, sCD14, in serum (27). The results from the present study suggest for the first time that ingestion of probiotics may affect serum sCD14 in infancy furnishing one mechanism by which probiotics might exert their effects. An overall trend for an increase in serum sCD14 concentrations during the first year of life, which reached statistical significance at 12 mo of age was observed.
Stimulation of CD14 and TLR2 by microbial products has been demonstrated to induce TGF-β production in human colonic cell lines (28) and probiotic strains of lactobacilli have been reported to induce the development of T cells producing TGF-β in vitro (29). In addition, there are data to suggest that CD14 is required for lactobacilli to induce a response (30). Taken together, these results may be interpreted to suggest that microbial stimulation in the intestine by probiotics leads to augmented TGF-β2 responses both systemically and on the mucosae as a result of enhanced signalling through CD14.
The results of the present study provide insight to the mechanisms through which probiotics may promote immunologic maturation in infancy. Supplementation with the probiotics Lactobacillus GG and Bifidobacterium lactis Bb-12 starting at the time of introduction of cow's milk in the infant's diet increased protective cow's milk-specific IgA responses. Our results further suggest that this might in part have been achieved via stimulation of the innate immune system through sCD14.
Abbreviations
- ELISPOT:
-
enzyme-linked immunospot assay
References
Macpherson AJ, Uhr T 2004 Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303: 1662–1665
Stavnezer J 1995 Regulation of antibody production and class switching by TGF-beta. J Immunol 155: 1647–1651
Petitprez K, Khalife J, Cetre C, Fontaine J, Lafitte S, Capron A, Grzych JM 1999 Cytokine mRNA expression in lymphoid organs associated with the expression of IgA response in the rat. Scand J Immunol 49: 14–20
Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD 1990 Protective effect of breast feeding against infection. BMJ 300: 11–16
Cunningham AS, Jelliffe DB, Jelliffe EFP 1991 Breast-feeding and health in the 1980s: a global epidemiologic review. J Pediatr 118: 659–666
van Odijk J, Kull I, Borres MP, Brandtzaeg P, Edberg U, Hanson LA, Host A, Kuitunen M, Olsen SF, Skerfving S, Sundell J, Wille S 2003 Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 58: 833–843
Bernt KM, Walker WA 1999 Human milk as a carrier of biochemical messages. Acta Paediatr Suppl 430: 27–41
Kalliomäki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E 1999 Transforming growth factor-beta in breast milk: a potential regulator of atopic disease at an early age. J Allergy Clin Immunol 104: 1251–1257
Jones CA, Holloway JA, Popplewell EJ, Diaper ND, Holloway JW, Vance GH, Warner JA, Warner JO 2002 Reduced soluble CD14 levels in amniotic fluid and breast milk are associated with the subsequent development of atopy, eczema, or both. J Allergy Clin Immunol 109: 858–866
Martin R, Langa S, Reviriego C, Jiminez E, Marin ML, Xaus J, Fernandez L, Rodriguez JM 2003 Human milk is a source of lactic acid bacteria for the infant. J Pediatr 143: 754–758
Beerens H, Romond C, Neut C 1980 Influence of breast-feeding on the bifid flora of the newborn intestine. Am J Clin Nutr 33: 2434–2439
Liepke C, Adermann K, Raida M, Magert HJ, Forssmann WG, Zucht HD 2002 Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur J Biochem 269: 712–718
Hanifin JM 1991 Atopic dermatitis in infants and children. Pediatr Clin North Am 38: 763–789
Isolauri E, Turjanmaa K 1996 Combined skin prick and patch testing enhances identification of food allergy in infants with atopic dermatitis. J Allergy Clin Immunol 97: 9–15
Isolauri E, Virtanen E, Jalonen T, Arvilommi H 1990 Local immune response measured in blood lymphocytes reflects the clinical reactivity of children with cow's milk allergy. Pediatric Res 28: 582–586
Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H 1992 Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res 32: 141–144
de Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J 2005 Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr 44: 406–413
Forrest BD 1988 Identification of an intestinal immune response using peripheral blood lymphocytes. Lancet 1: 81–83
Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E 2001 Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357: 1076–1079
Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E 2003 Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361: 1869–1871
Weiner HL 2001 Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect 3: 947–954
Rautava S, Kalliomäki M, Isolauri E 2002 Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol 109: 119–121
Newberry RD, Stevenson WF, Lorenz RG 1999 Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat Med 5: 900–906
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R 2004 Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241
Jin Y, Gupta D, Dziarski R 1998 Endothelial and epithelial cells do not respond to complexes of peptidoglycan with soluble CD14 but are activated indirectly by peptidoglycan-induced tumor necrosis factor-alpha and interleukin-1 from monocytes. J Infect Dis 177: 1629–1638
Backhed F, Meijer L, Normark S, Richter-Dahlfors A 2002 TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14. Cell Microbiol 4: 493–501
Labeta MO, Durieux JJ, Fernandez N, Herrmann R, Ferrara P 1993 Release from a human monocyte-like cell line of two different soluble forms of the lipopolysaccharide receptor, CD14. Eur J Immunol 23: 2144–2151
Yoshioka T, Morimoto Y, Iwagaki H, Itoh H, Saito S, Kobayashi N, Yagi T, Tanaka N 2001 Bacterial lipopolysaccharide induces transforming growth factor beta and hepatocyte growth factor through toll-like receptor 2 in cultured human colon cancer cells. J Int Med Res 29: 409–420
von der Weid T, Bulliard C, Schiffrin EJ 2001 Induction by a lactic acid bacterium of a population of CD4(+) T cells with low proliferative capacity that produce transforming growth factor beta and interleukin-10. Clin Diagn Lab Immunol 8: 695–701
Karlsson H, Hessle C, Rudin A 2002 Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect Immun 70: 6688–6696
Acknowledgements
The authors thank Sari Laksio, R.N., for caring and providing for the infants in the study. We also thank Satu Sivula and Etta-Liisa Väänänen for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the Microbes and Man Research Program, Academy of Finland, and the Sigrid Juselius Foundation.
Rights and permissions
About this article
Cite this article
Rautava, S., Arvilommi, H. & Isolauri, E. Specific Probiotics in Enhancing Maturation of IgA Responses in Formula-Fed Infants. Pediatr Res 60, 221–224 (2006). https://doi.org/10.1203/01.pdr.0000228317.72933.db
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000228317.72933.db
This article is cited by
-
Probiotic Supplementation for Prevention of Atopic Dermatitis in Infants and Children: A Systematic Review and Meta-analysis
American Journal of Clinical Dermatology (2019)
-
Sieving through gut models of colonization resistance
Nature Microbiology (2018)
-
Current Status of Potential Therapies for IgE-Mediated Food Allergy
Current Allergy and Asthma Reports (2018)
-
Dietary supplementation with Bifidobacterium longum subsp. infantis (B. infantis) in healthy breastfed infants: study protocol for a randomised controlled trial
Trials (2016)
-
Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood
Pediatric Research (2016)