Abstract
Pulmonary vascular development requires precise temporal and spatial expression of vascular endothelial growth factor-A (VEGF-A). Diminished expression of VEGF-A in preterm infants may contribute to the pathophysiology of respiratory distress syndrome. Because exogenous replacement of VEGF-A has been proposed as a therapeutic for respiratory distress syndrome, we used conditional activation of VEGF-A in bronchial epithelial cells to assess the effects of increase of VEGF-A on lung morphogenesis and survival in the developing mouse. Increased expression of VEGF-A in late stages of gestation was lethal at birth. Although born alive, the pups remained cyanotic and failed to establish respiration. Vascular and epithelial morphology of the main bronchus and primary and secondary bronchi were altered with neovascularization of the mucosal folds and partial obstruction of the conducting airways. Erythrocytes were observed in the pulmonary interstitium and in intra-alveolar spaces, indicating vascular leak. Increased diameter of pulmonary arteries and angioectatic structures were observed in VEGF-expressing mice. Bronchial expression of VEGF-A alters late-stage morphogenesis of conducting airways and primary bronchial arteries and causes respiratory failure at birth.
Similar content being viewed by others
Main
Recent studies of lung development demonstrate a direct relationship between the development of airways and accompanying vascular network. Several lines of evidence suggest that inadequate pulmonary vascular development may lead to underdeveloped airways and gas exchange units within the lung (1,2). Immaturity of pulmonary structures and function in preterm infants is directly related to the high morbidity and mortality associated with respiratory distress syndrome (RDS) in the United States. Recent work suggests that impaired production of vascular endothelial growth factor (VEGF) in lung leads to vascular damage and subsequent inadequate surfactant production and diminished lung development (3,4). In a model of RDS in premature baboons, VEGF and VEGF receptor R1 were reduced (4). In a recent study, Compernolle et al. (5) reported that therapeutic replacement of VEGF-A rescued preterm mice from respiratory distress without adversely effecting vascular permeability.
VEGFs are a family of protein effectors that influence endothelial cell behavior. Among these factors, VEGF-A is known to play an essential role in vascular development (6,7). VEGF-A regulates endothelial cell specification, proliferation, and migration and tubule formation. The temporal and spatial expression pattern of VEGF-A isoforms in the developing lung suggests that it is critical to the coordinated morphogenesis of airway and endothelium (8–10). Deletion of heparin-binding isoforms VEGF164 and VEGF188 attenuates pulmonary vascular development and results in fewer alveolar gas-exchange units (1). In the developing mouse lung, the heparin-binding VEGF-A isoforms are expressed after embryonic day 14.5 (E14.5) predominantly in peripheral airway epithelial cells with low or no expression in the conducting airways. Increased VEGF-A expression by peripheral airway epithelial cells during mid-lung organogenesis disrupts vascular net assembly and arrests branching morphogenesis (10). In contrast, ectopic expression of VEGF-A by epithelial cells of the conducting airways during the same period induced atypical neovascularization of the mucosal folds of the large airways (10). Treatment of neonatal rats with an inhibitor of VEGF receptor R2 disrupted postnatal lung development (11). Together, these data demonstrate that normal lung development requires appropriate spatial expression of VEGF-A in a narrow dose range.
VEGF-A also regulates functions of fully developed vascular systems, including response to injury and tumors. VEGF-A was originally characterized by its ability to increase flux of plasma proteins across endothelial microvessels (12). Dvorak et al. (13) suggested that this microvascular permeability, involving the loosening of intraendothelial junctions, plays a role in endothelial cell “sprouting” during angiogenesis. However, hyperpermeability leads to flow of fluid, protein, and cells across the vessel wall. In adult mouse lung, increased expression of VEGF induces pulmonary vascular permeability and causes edema (14). Therefore, the effects of VEGF on lung development in late gestation and potential effects on vascular leak merit study before VEGF is considered as a potential therapeutic for RDS or other pulmonary syndromes in neonates. We have conditionally expressed VEGF in bronchial epithelial cells in late gestation and analyzed lung morphogenesis.
METHODS
Transgenic mice.
All procedures and handling of transgenic mice were reviewed and approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Research Foundation. Generation and analysis of CCSPrtTA, (tetO)7VEGF and bitransgenic CCSPrtTA/(tetO)7VEGF were previously described (10). Briefly, in the activator transgenic line, the reverse tetracycline transactivator (rtTA) has the 2.3-kb rat Clara cell secretory protein (CCSP) promoter. In the presence of doxycycline, the rtTA directs expression of transgenes under control of the tet operator (tetO) primarily to proximal, conducting airway epithelium (15). Without doxycycline, there is no significant induction or “leakiness” of tetO transgenes (10), including VEGF164 (Fig. 1C and D). The flk.LacZ animals were purchased from Jackson Laboratories (Bar Harbor, ME). In the experiments described here, bitransgenic animals were generated by breeding CCSPrtTA mice to mice either hemizygous or homozygous for (tetO)7VEGF. Pregnancies were staged by detection of a vaginal plug on the day designated as E0.5. For inducing embryonic VEGF164 transgene expression, pregnant animals were given doxycycline-containing food (625 mg/kg; HarlanTekland, Madison, WI) for the designated periods. At E18.5, pregnant dams were killed by CO2 inhalation, and the fetuses were removed by hysterotomy.
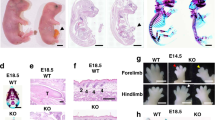
Morphology of conducting airways altered by VEGF164. Lung morphology of E18.5 lungs for nontransgenic (A, B, and G) and CCSPrtTA/(tetO)7VEGF without doxycycline treatment (C and D) and with doxycycline treatment from E10.5 to E18.5 (E, F, and H) or from E16.5 to E18.5 (I and J) was assessed after staining with haematoxylin and eosin. Bronchi (br), alveoli (al), and primary arteries (ar) and veins (ve) are identified. Primary bronchi were indistinguishable for nontransgenic (A and B) and bitransgenic lung (C and D) from embryos that were not exposed to doxycycline. In the bronchi, small regularly spaced mucosal folds evaginated into the airway lumen. On VEGF164 expression from either E10.5 to E18.5 (E and F) or E16.5 to E18.5 (I and J), mucosal folds were enlarged (*) and irregular. The enlarged mucosal folds partially obstructed the conducting airways (F and I). Distal lung morphology and airway branching were similar in nontransgenic (G) and CCSPrtTA/(tetO)7VEGF (H) after doxycycline treatment from E10.5 to E18.5. Bar = 100 mm.
Histology, immunohistochemistry, and in situ hybridization analysis.
Whole embryos or isolated lungs were fixed in 4% paraformaldehyde and paraffin-embedded as described (16). For histology, 4-μm sections were stained with haematoxylin/eosin. The antibodies (Ab) used for immunohistochemistry were generated to PECAM-1 (1:500, Ab Mec13.3; BD PharMingen, San Diego, CA); smooth muscle α actin (1:15,000, Ab 4A1; Sigma Chemical Co., St. Louis, MO); pro-surfactant protein C (SP-C; 1:1000, Ab3428; Chemicon, Temecula, CA), pro-surfactant protein B (SP-B; 1:1500, Chemicon), CCSP (1:5000, a rabbit polyclonal; a gift of Dr. Barry Stripp, University of Pittsburgh); FOXj1 (1:4000, rabbit polyclonal; a gift of Dr. Robert Costa, University of Illinois); and cytokeratin (1:500, Ab PCK-26; Sigma Chemical Co.). Biotinylated secondary antibodies and the streptavidin-biotin-peroxidase detection system were from Vector Laboratories (Burlingame, CA). Endothelial-specific lectin staining (17) was done with FITC-conjugated GSL B4 isolectin (Vector Laboratories) with 10 mM of CaCl2 and 20 mM of MgCl2. For analyzing proliferation, pregnant dams were given i.p. injections of bromodeoxyuridine (BrdU) at 1 μg/g weight, and embryos were collected 2 h later. BrdU-labeled cells were detected with an immunostaining kit from Zymed (San Francisco, CA). In situ hybridization analysis was performed using [35S]UTP-labeled riboprobe for murine VEGF-A as previously described (16).
Whole-mount analysis.
Lungs of CCSPrtTA/(tetO)7VEGF/flk.LacZ embryos were dissected at E17.5, fixed in 0.2% glutaraldehyde and 2% paraformaldehyde for 2 h, and incubated with β-D galatactopyranoside substrate X-gal to detect β-galactosidase activity (18). After washing in PBS, lungs were examined with an Olympus inverted microscope, and the images were recorded with a digital MagnaFire camera.
Lung wet/dry weight determination.
After the mice were killed, embryos were decapitated and thoroughly bled out. Lungs were removed and dissected away from heart and thymus. Lungs were immediately weighed, dried in a desiccating oven at 80°C for 24 h, and weighed again. Water content was measured as the ratio of wet/dry weight (14).
RESULTS
Late embryonic expression of VEGF in conducting airways is lethal.
In this study, the CCSP promoter was used to direct ectopic transgene expression from epithelial cells of the proximal, conducting airways. In normal lung, the bronchial epithelium is not a major source of VEGF-A (16). We previously used bitransgenic mice with VEGF164 expression under conditional control of the CCSP promoter to analyze the effect of ectopic VEGF-A on early bronchial development up to E16.5 (10). When CCSPrtTA/(tetO)7VEGF mice were activated with exposure to doxycycline from E10.5 to E16.5, there was an ∼2.5-fold increase in VEGF-A levels in lung (10). Bronchial expression of VEGF-A during this period, the embryonic and pseudoglandular stages of development, led to hypervascularization and altered morphology of the conducting airways. There was no morphologic change in the large arteries and veins that accompany the conducting airways. Distal vascular and alveolar development was also unaffected (10).
For examining the effects of bronchial expression of VEGF-A on late-stage development, doxycycline treatment was extended from E10.5 until E18.5. Without doxycycline treatment, lung morphology of nontransgenic (Fig. 1A and B) and bitransgenic (Fig. 1C and D) animals was indistinguishable. This demonstrates minimal induction or “leak” of VEGF164 in the absence of doxycycline. With VEGF164 induction from E10.5 to E18.5 (Fig. 1E and F), both conducting airways and the accompanying primary vessels were dysplastic and the morphology of the proximal lung was highly disrupted. Mucosal folds of the bronchi were enlarged and partially obstructing the conducting airways. Alterations in airway morphology at E18.5 were similar for lungs from animals that were treated from E10.5 to E16.5 (10). However, analysis at E18.5 showed that VEGF164 increased the diameter of primary blood vessels. At E16.5, these vessels were similar in doxycycline-treated nontransgenic and bitransgenic lung. Airway epithelial development and alveolar branching in distal lung seemed unaltered compared with nontransgenic (Fig. 1G and H) at E18.5.
General morphology of the proximal lung in mice is well established by the saccular stage, beginning at E17 (19). In this study, VEGF164 expression was initiated early in lung development at E10.5 and sustained into the saccular stage at E18.5. The altered morphology, therefore, may reflect the effects of VEGF164 on early developmental programs, whereas late-stage development is less susceptible to ectopic VEGF164. For testing this possibility, bronchial induction of VEGF164 was delayed until E16.5. Late embryonic lung development was still susceptible to VEGF164. Analysis of lung morphology at E18.5 again showed highly dysplastic primary vessels and conducting airways (Fig. 1I and J). Enlarged mucosal folds with capillary-like vessels were also detected with VEGF164 induction for only 24 h, E17.5 to E18.5 (A.L.A., personal observation). However, with this 1-d protocol, the phenotype was variable for bitransgenic embryos within the same litter. Systemic embryonic concentrations of doxycycline may not have reached saturation within 1 d, leading to variable phenotype.
The effects of VEGF-A on lung function were evaluated by monitoring the survival of pups after VEGF-A induction. Bitransgenic animals, hemizygous for both the CCSPrtTA (C+/−) and the (tetO)7VEGF (V+/−) transgenes, were treated with doxycycline from either E10.5 until birth or from E16.5 until birth. Doxycycline-treated bitransgenic pups from both treatment groups died within 24 h, whereas untreated bitransgenic animals had 100% survival (Table 1). Bitransgenic CCSPrtTA/(tetO)7VEGF pups were born alive but remained cyanotic with labored breathing. On dissection, the lungs of bitransgenic animals were hemorrhagic. These data demonstrate that bronchial expression of VEGF164 as late as E16.5 causes neonatal death.
Ectopic VEGF-A alters late-stage bronchial development.
For identifying the cause of death after VEGF-A expression in conducting airways, nontransgenic and CCSPrtTA/(tetO)7VEGF animals were treated from E16.5 to E18.5 with doxycycline and analyzed at E18.5. Irregular mucosal evaginations of the epithelium into the airway lumen in primary and secondary bronchi and the main stem bronchus were observed in the lungs of CCSPrtTA/(tetO)7VEGF mice (Fig. 2). In situ hybridization analysis for VEGF-A mRNA was used to analyze endogenous and transgene expression. At E18.5, endogenous VEGF-A mRNA was found in distal airways but not in conducting airways of single transgenic CCSPrtTA or (tetO)7VEGF or nontransgenic littermates (Fig. 2A). In doxycycline-treated CCSPrtTA/(tetO)7VEGF mice (Fig. 2B), VEGF-A mRNA was also detected in the main bronchus and primary and secondary conducting airways.
VEGF induces neovascularization of mucosal folds of main bronchus. In situ hybridization analysis demonstrates VEGF-A expression in lung of nontransgenic (A) and CCSPrtTA/(tetO)7VEGF (B) animals after doxycycline treatment from E16.5 to E18.5. Normal expression of VEGF mRNA in nontransgenic littermates was in distal epithelium but not bronchi (A). After doxycycline induction, in CCSPrtTA/(tetO)7VEGF lung, VEGF-A mRNA was also detected in bronchi (B). Immunohistochemistry for PECAM (C and D) and smooth muscle α actin (E and F) was used to identify endothelial cells and smooth muscle cells, respectively, in consecutive sections. Few endothelial cells were detected in mucosal folds of nontransgenic lung (C). In CCSPrtTA/(tetO)7VEGF lungs, endothelial cells formed blood-filled, capillary-like vessels in each mucosal fold (D). The capillary-like vessels were not accompanied by smooth muscle α actin–positive cells (F). (G and H) Green fluorescence indicates endothelial cells labeled with FITC GSL B4, red indicates epithelial cells labeled with TxR-Ab to cytokeratin, and yellow indicates autofluorescence of red blood cells. In nontransgenic lung at E18.5, endothelial cells were separated from bronchial epithelial cells by several layers of mesenchymal cells (G, arrow). In bitransgenic lung, endothelial cells directly contact the bronchial epithelial cells (H, arrows). Magnification is the same in panels A–F and indicated in F with scale bar = 50 mm; magnification is the same in panels G–H, indicated in H with scale bar = 20 mm.
In CCSPrtTA/(tetO)7VEGF lung (Fig. 1I and J), the primary and secondary bronchi were enlarged compared with control, nontransgenic lung (Fig. 1A–D). However, the diameter of the main bronchus of CCSPrtTA/(tetO)7VEGF lung (Fig. 2C) was normal. In normal mouse lung, the main bronchi are encircled by a loose network of small capillaries detected by immunostaining to PECAM. Capillary endothelial cells do not directly contact the epithelium and are rarely detected in the epithelial folds that protrude into the airway (Fig. 2C). Several layers of smooth muscle cells continuously encircle each bronchus (Fig. 2E). In CCSPrtTA/(tetO)7VEGF lung, hypervascularization of the bronchus was observed. The epithelial folds contained capillary-like vessels (Fig. 2D). The abnormal vessels frequently had lumens that contained red blood cells (Fig. 2H). Smooth muscle α actin was not associated with the capillary-like vessels (Fig. 2E and F), indicating the absence of smooth muscle cells in the mucosal folds. The continuous layer of smooth muscle cells around the bronchi seemed unaffected. Endothelial cells, identified with FITC-conjugated lectin GSL B4, were in close contact with the airway epithelium (Fig. 2E, arrows). However, the morphology of individual endothelial cells seemed typical of normal microvascular cells as shown by FITC-GSL B4 staining in Fig. 2G and H.
VEGF164 perturbs proximal lung vascular morphogenesis.
Analysis of lung thin sections showed several types of alterations in vascular development after VEGF164 expression in conducting airways (Fig. 3). The diameter of primary vessels positioned along conducting airways was increased (Fig. 3A and B). Arteries were more severely affected than veins. Arteries are more closely aligned with conducting airways than veins, thus closer to the source of VEGF164 transgene expression. As with early bronchial induction of VEGF164 (10), the vascular patterning of distal lung was unaffected (Fig. 3A and B, inset). Immunohistochemistry for PECAM (Fig. 3B and D) and smooth muscle α actin (Fig. 3F) suggested that cellular composition of the enlarged primary vessels was typical of normal arteries (Fig. 3A, C, and E). There was a single layer of endothelial cells surrounded by several layers of smooth muscle cells in the primary vessels. Secondary vascular tubules developed into large angioectatic structures that contained red blood cells in some of the affected mice. The walls of these abnormal structures were lined by endothelial cells (Fig. 3I).
Primary blood vessel morphology altered by VEGF164. Nontransgenic (A, C, E, G, and M) and CCSPrtTA/(tetO)7VEGF (B, D, F, H, I, J, K, and L) animals were treated with doxycycline from E16.5 to E18.5. PECAM immunostaining (A–D) shows vascular patterning. Arteries (ar) close to bronchi (br) expressing VEGF164 were enlarged in CCSPrtTA/(tetO)7VEGF lung. In both nontransgenic (C and E) and CCSPrtTA/(tetO)7VEGF (D and F) lung, the arteries were composed of a single layer of PECAM-positive endothelial cells surrounded by several layers of α actin–positive smooth muscle cells. In several CCSPrtTA/(tetO)7VEGF lungs, ectatic structures lined with PECAM-positive endothelial cells were detected (I). BrdU analysis showed no difference in proliferation for cells of the enlarged arteries (outlined in blue in J) or the angioectatic structure (K, arrows). Consecutive sections are shown in panels D, F, and J and in panels I and K. The pulmonary vasculature was examined by whole-mount analysis of E17.5 lung from flk.LacZ (G) and CCSPrtTA/(tetO)7VEGF.flk.LacZ (H) animals. Arteries (ar) along the length of the conducting airway bronchi (br) were enlarged. Compare arteries indicated by arrows in G (nontransgenic) and H (CCSPrtTA/(tetO)7VEGF). Using immunofluorescence, many areas with intersitial and intra-alveolar red blood cells were identified in CCSPrtTA/(tetO)7VEGF lung (L, arrows) but few in nontransgenic lung (M, arrows). In L and M, endothelial cells were identified with FITC-GSL B4, epithelial cells with TxRed-Ab to cytokeratin, and red blood cells with yellow autofluorescence. Scale is indicated in panels A–K, L, M. Magnification in panels G and H is 7×.
Enlarged vessels were also detected on whole-mount preparations of lung from CCSPrtTA/(tetO)7VEGF/flk.LacZ animals. Endothelial cells of flk.LacZ animals (18,20) express the reporter gene β-galactosidase (LacZ) under control of the promoter for the VEGF receptor flk (or VEGFR2), providing visualization of the vascular system in whole-mount analysis. CCSPrtTA/(tetO)7VEGF were bred to flk.LacZ animals, and the pregnant dams were treated with doxycycline from E10.5 to E17.5. Embryonic lungs were collected at E17.5, sectioned at 100 μm, and stained with β-galactosidase substrate. Compared with an flk.LacZ littermate (Fig. 3G), blood vessels in the lungs of CCSPrtTA/(tetO)7VEGF/flk.LacZ animals were intensely stained and enlarged (Fig. 3H)
Endothelial proliferation can be induced in vivo by ectopic expression of VEGF-A (21). The increased diameter of primary vessels in the lungs of bitransgenic animals may be the consequence of increased proliferation of endothelial cells. Dams received an injection of BrdU 2 h before collection of embryos. Figure 3J shows BrdU staining of the enlarged vessel shown in sequential sections in Fig. 3D and F. Figure 3K shows BrdU staining for tissue surrounding the angioectatic structure in Fig. 3I. BrdU incorporation rates were not altered by increased VEGF-A. This analysis demonstrates that endothelial proliferation at E18.5 was not detectably altered by increased expression of VEGF-A.
Immunofluorescence analysis revealed red blood cells (detected as yellow autofluorescence in Fig. 3L and M) present in both interstitial and intra-alveolar spaces, indicating compromised vessel and/or capillary integrity. Gross hemorrhage was detected only in the lungs of doxycycline-treated CCSPrtTA/(tetO)7VEGF animals (Fig. 3L) but not in untreated bitransgenic or doxycycline-treated, nontransgenic pups (Fig. 3M).
The presence of pulmonary hemorrhage suggests that VEGF-A increased vascular permeability. In neonates and adults, increased vascular permeability leads to leakage of water, serum proteins, and blood cells measured by comparison of lung wet weight with dry weight (14). No significant differences in wet/dry weight were observed at E18.5 after 2 d of VEGF164 induction. For untreated animals (n = 11), the mean wet to dry weight ratio was 7.67 ± 0.75 and for doxycycline-treated bitransgenics (n = 23) was 7.71 ± 0.89 (p = 0.3). Methods that traditionally have been used to measure edema, including determination of the ratio of wet to dry weight, may not be appropriate for analysis of vascular leak before birth, as lungs are filled with amniotic fluid.
VEGF164 alters bronchial epithelial morphology but not differentiation. Immunohistochemistry was used to examine epithelial cell types in the affected bronchi. Figure 4 compares immunostaining patterns of E18.5 lung from nontransgenic and CCSPrtTA/(tetO)7VEGF164 animals after 2 d of VEGF164 induction. Immunostaining for CCSP, a bronchial cell marker (Fig. 4A and B), and Foxj1, a ciliated/bronchial cell marker (Fig. 4C and D), indicates that both bronchial and ciliated epithelial cells are present in numbers similar in nontransgenic and CCSPrtTA/(tetO)7VEGF164 lung. However, the spatial relationship of the epithelial cell populations is disrupted. In distal lung, robust staining for pro–SP-C and low-level staining for SP-B was indistinguishable between nontransgenic and CCSPrtTA/(tetO)7VEGF164 lung. This showed that despite the altered morphology, epithelial cell differentiation in the conducting airways was not affected by VEGF164.
Bronchial epithelial morphology but not differentiation altered by VEGF164. E18.5 lungs from nontransgenic (A, C, E, and G) and CCSPrtTA/(tetO)7VEGF (B, D, F, and H) animals with doxycycline-induced VEGF164 from E16.5 to E18.5. Immunostaining for CCSP (A and B) identified bronchial epithelial cells and Foxj1 (C and D) identified both bronchial and ciliated epithelial cells. Distal airway epithelial cells were identified by immunostaining for pro–SP-C (E and F) and SP-B (G and H). Original magnification in all panels is 20×, indicated in H with scale bar = 50 mm.
DISCUSSION
During late-stage mouse lung organogenesis, development of the conducting airways and primary pulmonary vascular system are strongly influenced by VEGF164. VEGF164 induces atypical neovascularization in the epithelial folds and surrounding interstitial tissue of the main bronchus and conducting airways. Neovascularization was present with VEGF164 induction as late as E17.5. The altered morphology of the conducting airways may simply reflect accommodation of the neovascularization. The resulting airway obstruction likely contributes to the respiratory distress and mortality of the neonates. Bronchial expression of VEGF164 also altered the morphology of primary arteries that closely accompany the conducting airways. The arteries were enlarged in diameter, but proliferation of both endothelial cells and vascular smooth muscl cells was unchanged. This demonstrates that vessels that accompany the conducting airways remain responsive to VEGF-A through the late canalicular and saccular stages of lung morphogenesis. Preterm infants who are born at <32 wk of gestation have lungs that match this stage of development.
The new capillary-like vessels form within the pulmonary interstitium that does not normally support vascular development and may not provide the appropriate physical support, matrix, and growth factors. We propose that the inappropriate microenvironment results in poor vessel wall integrity and extravascular blood within the lung. Thurston et al. (22) reported similar effects on dermal microvessels on increased expression of VEGF. Using virally transduced myoblasts to constitutively express VEGF164, Ozawa et al. (23) showed that microenvironment and not total dose determines the threshold for angiogenesis of normal versus aberrant vessels.
Effects of VEGF-A on vascular permeability may be different in adult and fetal lung. The developmental stage, VEGF-A dose, and route of delivery may determine the extent of vascular leak and edema. In adult, systemic delivery of VEGF-A induces vascular permeability, leading to leakage of water and serum proteins (24). Kaner et al. (14) showed that a 8- to 9-fold induction of VEGF-A through intratracheal administration of an adenovirus vector in adult mice induces measurable pulmonary edema. Edema, measured by wet/dry weight and lung permeability to albumin, was detected 1 d after adenoviral delivery of VEGF-A, and lung histology showed interstitial and intra-alveolar presence of edema. In contrast, conditional embryonic activation of VEGF164 in CCSPrtTA/(tetO)7VEGF animals increased VEGF-A in embryonic lung lysates only 2.5-fold (10). The lower dose of ectopic VEGF-A in CCSPrtTA/(tetO)7VEGF animals may be insufficient for induction of pulmonary edema. In addition, neonatal lung may be less susceptible to VEGF-induced edema than adult lung.
VEGF164 and the larger isoform VEGF188 bind heparan sulfate on cells and in the extracellular matrix, limiting diffusibility. In our bitransgenic models, VEGF164 is induced within the tissue microenvironment of either peripheral (10) or proximal lung. As we reported (10), using SPCrtTA and doxycycline to induce VEGF164 expression by peripheral airway epithelial cells disrupts capillary network assembly, smooth muscle pattern formation, and distal airway branching but has no effect on conducting airways. Conversely, the effects of expression of VEGF164 by CCSP-expressing cells of the bronchial epithelium are limited to proximal lung. Distal lung seems unaltered. CCSP-driven expression of VEGF164 does not alter smooth muscle cell morphology of either affected bronchi or large arteries. These smooth muscle cell populations may be further differentiated and less responsive to VEGF164 than smooth muscle cells of distal lung. The unique microenvironment of proximal and distal lung may also play a role in smooth muscle cell responsiveness.
Compernolle et al. (5) tested the effect of VEGF-A on lung development and function. VEGF-A administered as a bolus to premature mice prolonged survival for several hours. Alveolar angiogenesis, vascular leakage, or bronchial edema was not detected in the short duration of the study. Compernolle et al. (5) suggested that this prolonged survival resulted from increased production of surfactant proteins as a consequence of direct effect of VEGF on bronchial epithelial cells. In contrast, with sustained tissue-generated VEGF-A expression during the same stage of lung development, we found neovascularization in the conducting airways and evidence of vascular leakage but not significant bronchial edema. We found no evidence of altered production of SP-C, SP-B, or CCSP.
We previously reported VEGFR2 expression of embryonic mouse lung (10). We found that immunostaining for VEGFR2 was coincident with that of PECAM-1, an endothelial marker restricted to blood vessels and capillaries. Neither distal alveolar epithelial cells nor bronchial epithelial cells were immunopositive for VEGFR2. Therefore, we propose that changes in bronchial epithelial morphology are an indirect result of neovascularization into the mucosal folds. To compensate for the new vessels, epithelial patterning is disrupted. However, epithelial differentiation and production of surfactant proteins seem unchanged.
CONCLUSION
In summary, conditional activation of VEGF164 expression in bronchial epithelial cells in late gestation caused respiratory failure at birth. Distal lung morphology was unaffected, suggesting that respiratory function at birth was compromised as a result of the neovascularization and altered morphology of the conducting airways. We propose that abnormal capillary-like vessels within the bronchial folds contributed to pulmonary hemorrhage and death. These data suggest that intratracheal administration of VEGF-A for premature infants for RDS should be approached cautiously. Careful determination of VEGF-A dose and type and length of administration will be critical for efficacious therapy without increases in vascular leak or hemorrhage.
Abbreviations
- Ab:
-
antibodies
- BrdU:
-
bromodeoxyuridine
- CCSP:
-
Clara cell secretory protein
- E:
-
embryonic day
- GSL B4:
-
Griffonia simplicifolia lectin B4
- R:
-
receptor
- RDS:
-
respiratory distress syndrome
- rtTA:
-
reverse tetracycline responsive transactivator
- SP-C:
-
surfactant protein C
- SP-B:
-
surfactant protein B
- tetO:
-
tetracycline operator
- VEGF:
-
vascular endothelial growth factor
References
Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D'Amore PA, DeMello DE 2002 Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol 27: 194–203
Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH 2000 Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607
Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S 2001 Pulmonary vascular endothelial growth factor and flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164: 1981–1987
Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H 2002 Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 282: L811–L823
Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P 2002 Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710
Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW 1996 Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442
Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A 1996 Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439
Park JE, Keller GA, Ferrara N 1993 The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 4: 1317–1326
Ng YS, Rohan R, Sunday ME, Demello DE, D'Amore PA 2001 Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn 220: 112–121
Akeson AL, Greenberg JM, Cameron JE, Thompson FY, Brooks SK, Wiginton D, Whitsett JA 2003 Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev Biol 264: 443–455
Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH 2002 Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol 283: L555–L562
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF 1983 Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983–985
Dvorak HF, Brown LF, Detmar M, Dvorak AM 1995 Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039
Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG 2000 Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 22: 657–664
Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA 1992 cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 267: 14703–14712
Greenberg JM, Thompson FY, Brooks SK, Shannon JM, McCormick-Shannon K, Cameron JE, Mallory BP, Akeson AL 2002 Mesenchymal expression of vascular endothelial growth factors D and A defines vascular patterning in developing lung. Dev Dyn 224: 144–153
Ponder BA, Festing MF, Wilkinson MM 1985 An allelic difference determines reciprocal patterns of expression of binding sites for Dolichos biflorus lectin in inbred strains of mice. J Embryol Exp Morphol 87: 229–239
Schachtner SK, Wang Y, Scott Baldwin H 2000 Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol 22: 157–165
Burri PH 1992 Intussusceptive microvascular growth, a new mechanism of capillary network formation. EXS 61: 32–39
Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A 1999 Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol 212: 307–322
LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N 2003 Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science 299: 890–893
Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM 1999 Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514
Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM 2004 Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest 113: 516–527
Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, Stiber JA, Lobo AD, Hunsberger S, Guetta E, Epstein SE, Unger EF 1996 Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation 94: 1074–1082
Acknowledgements
We thank Tim Mead for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by National Heart, Lung, and Blood Institute Grant HL-067807 (A.L.A.), SCOR 5P5OHL56837 (J.A.W.), and American Lung Association Grant CI-31-N (T.D.L.).
Rights and permissions
About this article
Cite this article
Akeson, A., Cameron, J., Le Cras, T. et al. Vascular Endothelial Growth Factor-A Induces Prenatal Neovascularization and Alters Bronchial Development in Mice. Pediatr Res 57, 82–88 (2005). https://doi.org/10.1203/01.PDR.0000148070.89006.3F
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000148070.89006.3F
This article is cited by
-
Interactions between genes altered during cardiotoxicity and neurotoxicity in zebrafish revealed using induced network modules analysis
Scientific Reports (2023)
-
The BPD trio? Interaction of dysregulated PDGF, VEGF, and TGF signaling in neonatal chronic lung disease
Molecular and Cellular Pediatrics (2017)
-
Deleted in malignant brain tumors 1 (DMBT1) elicits increased VEGF and decreased IL-6 production in type II lung epithelial cells
BMC Pulmonary Medicine (2015)
-
Regulation of lung development and regeneration by the vascular system
Cellular and Molecular Life Sciences (2015)