Abstract
Studies of ventilator-associated lung injury in adult experimental animal models have documented that high tidal volume (TV) results in lung injury characterized by impaired compliance and dysfunctional surfactant. Yet, there is evidence that, in neonates, ventilation with a higher than physiologic TV leads to improved lung compliance. The purpose of our study was to evaluate how lung compliance and surfactant was altered by high TV ventilation in the neonate. We utilized a new model (mechanically air-ventilated newborn rats, 4–8 d old), and used 40 or 10 mL/kg TV strategies. Age-matched nonventilated animals served as controls. In all animals, dynamic compliance progressively increased after initiation of mechanical ventilation and was significantly greater than basal values after 60 min (p < 0.01). Lung lavage total surfactant with both TV strategies (p < 0.05) and the large aggregate fraction (only in TV = 40 mL/kg; p < 0.01) were significantly increased by 60 min of mechanical ventilation, compared with control animals. Ventilation with 40 mL/kg TV for 60 min adversely affected the lung surfactant surface-tension lowering properties (p < 0.01). After 180 min of ventilation with 40 mL/kg TV, the lung total surfactant content and dynamic compliance values were no longer distinct from the nonventilated animals' values. We conclude that, in the newborn rat, mechanical ventilation with a higher than physiologic TV increases alveolar surfactant content and, over time, alters its biophysical properties, thus promoting an initial but transient improvement in lung compliance.
Similar content being viewed by others
Main
A single stretch of alveolar type II cells causes an increase in cytosolic calcium resulting in stimulation of surfactant secretion for up to 30 min post stretch (1). In adult animals, this mechanism is operative after an increase in spontaneous breathing tidal volume (2, 3), but never demonstrated after short-term mechanical ventilation with a higher than physiologic tidal volume. Isolated adult rat lungs mechanically ventilated with a tidal volume four times higher than physiologic (25 mL/kg) for 1 h showed a decrease in lung compliance (4), which was associated with an increased conversion from LA to SA within the alveolar space (5). These latter changes likely accounted for the reduction in compliance, inasmuch as adequate biophysical activity of surfactant is dependent on a functional LA fraction.
The concept that high tidal volumes may have adverse effects has been consistently demonstrated in multiple animal models of adult ventilator-associated lung injury (6–8). This concept has driven a variety of strategies aimed at reducing tidal stretch, including permissive hypercapnia (9–11), as well as studies designed to limit pressure and volume ventilatory parameters associated with tidal stretch (12–14). Indeed, in adult ARDS, this approach has been vindicated with the demonstration that increased tidal volume ventilation is associated with worsened mortality in patients with ARDS (15, 16).
In the neonate, however, the response to short-term ventilation with higher tidal volumes appears to contrast with the adult. Ratjen et al. (17) studied the lung mechanical properties of healthy full-term infants subjected to a few inspiratory efforts with a tidal volume 75% greater than that observed during regular breathing (10 mL/kg). A significant improvement in total respiratory system compliance was observed, and was explained on the basis of recruitment of atelectatic lung units. Yet stretch-induced changes in surfactant synthesis/ release may account for this response because there is evidence that the fetal lung epithelial cells respond to stretch with an increase in surfactant production (18). Although lung recruitment strategies appear to be of clinical benefit in neonates (19), the specific effect of in vivo lung stretch on endogenous surfactant release and/or secretion in neonates has not been studied.
Thus, the purpose of this study was to evaluate the in vivo changes in total respiratory system compliance and the surfactant system in normal newborn rats after mechanical ventilation with 10 and 40 mL/kg tidal volume strategies. We hypothesized that ventilation with 10, but not 40 mL/kg tidal volume results in lung surfactant release and/or secretion and improved lung compliance.
METHODS
Animal preparation.
Newborn Sprague Dawley rats aged 5–8 d kept with their dam until the day of the experiment were used. The animals were sedated with an intraperitoneal injection of a mixture of xylazine and ketamine (8 and 80 mg/kg, respectively).
Following a small neck incision, a metal cannula (22 gauge, 2.5 cm in length) was placed in the trachea and firmly secured with a 4.0 silk suture to prevent air leak. The cannula was connected to the mechanical ventilator, after which the animal was paralyzed with pancuronium bromide (0.1 mg/kg i.m.). Chest electrodes were placed and the animal's ECG signal was acquired continuously (Hewlett Packard, Palo Alto, CA, U.S.A.) to monitor for cardiovascular stability. The animal was kept on a warm circulating water pad to maintain the body temperature constant at 37°C.
Institutional review.
All procedures involving animals were conducted according to criteria established by the Canadian Council for Animal Care. Approval for the study was obtained from the Animal Care Review Committee of the Hospital for Sick Children Research Institute.
Mechanical ventilation.
The animals were air-ventilated with a tidal volume of 10 or 40 mL/kg in a randomized manner with a rate of 60 breaths/min and 1:1 inspiratory/expiratory ratio for 60 min. A PEEP = 0 was applied to the animals ventilated with 40 mL/kg (n = 15). An additional four newborn animals were ventilated for a total of 180 min with a tidal volume of 40 mL/kg and PEEP = 0. The higher tidal volume with a PEEP = 0 ventilation strategy has been shown to result in lung injury in the adult rat (20).
The 10 mL/kg tidal volume group was placed on a PEEP of 2 cm H2O to avoid lung atelectasis (n = 9). Nonventilated newborn animals served as controls (n = 12). In pilot experiments, we collected at the end of the ventilation period mixed arterial/venous blood after decapitation for pH and blood gases measurements. The pH was 7.31 ± 0.09 and 7.55 ± 0.09 and the PCO2 was 58 ± 16 and 31 ± 9 torr for the 10 (n = 3) and 40 mL/kg ventilated animals (n = 3), respectively.
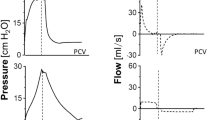
A custom-made mechanical ventilator, previously described, was used (21) Briefly, the ventilator incorporates a 24-cm-long capillary tube with a very small internal diameter (0.025 cm) placed between a high-pressure gas source and the animal. The animal is connected to the ventilator via a miniature Y piece (dead space, 0.02 mL) and plastic tubes of low compliance. One tube is for inspiration, the second one is for expiration, and the third is for connection to a pressure transducer in the ventilator for monitoring airway-opening pressure. The expiratory circuit is approximately linear in the flow ranges used and expired volume can be measured by integrating expiratory flow.
Total respiratory system dynamic compliance was measured by recording the ventilator tidal volume (measured as expired volume by the internal pneumotachometer) and peak inspiratory– PEEP (as measured by the internal pressure transducer). The ventilator pressure transducer and pneumotachometer measurements were validated after each experiment with a water manometer and calibrated volume syringe, respectively. Compliance measurements were either normalized to the animals' body weight, or expressed as a percentage of the first measurement obtained immediately after intubation (time zero).
Lung lavage and surfactant determination.
Immediately after being killed, all animals underwent a modified whole lung lavage, previously described for adult rats (22). Briefly, the lungs were infused with sterile 0.5 mL saline and the saline was withdrawn and reinfused two more times. This procedure was repeated a total of three times, and the combined volume of the total lavage was recorded. There were no differences in the total volume of saline infused or recovered after the lavage procedure among the experimental and control groups. Previous studies have shown that this lavage procedure removes more than 90% of the alveolar surfactant in both normal animals and those with acute lung injury (23).
The total lavage was centrifuged at 150 g for 10 min to yield a pellet containing cellular debris. The supernatant was then spun at 40,000 g for 15 min, yielding a supernatant that represented the small surfactant aggregate fraction. The 40,000-g pellet was suspended in 2 mL of 0.15 M saline and represented the large surfactant aggregate fraction.
To measure the total phospholipid pool size and individual surfactant aggregate phospholipid pool sizes, aliquots from the total lavage, 150-g pellet, LA, and SA fractions were organic solvent extracted and phospholipid-phosphorous levels in each of these extracts were determined by using the Duck-Chong phosphorous assay (24). Briefly, 100 μL of 10% (wt/vol) magnesium nitrate in methanol were added to the extracted lipids. After being dried, the samples were ashed in a fume hood on an electric rack for about 1 min. After 1 mL of 1 M HCl was added, the samples were reheated on a heating block while covered for 15 min at 95°C. After cooling, a 66-μL aliquot of each sample was added to individual wells of a 96-well plate along with 134 μL of a dye consisting of 4.2% ammonium molybdate in 4.5 M HCl with 0.3% malachite green (1:3 vol/vol). The absorbency of each sample was read at 650 nm by using a MKII Titretek Multiscan ELISA plate reader (Flow Laboratories Int., Switzerland) and compared with phosphorus reference standards on the same plate.
Measurement of the surfactant biophysical properties.
We used the captive bubble surfactometer technique previously described (25). Briefly, the captive bubble method is an in vitro technique developed for the determination of the surface tension, area, and volume of a bubble with a surfactant film at the air-water interface of the bubble. Changing the pressure within the closed chamber can alter the bubble size and, thus, the surface tension of any insoluble film at the bubble surface.
The instrument consists of a sample chamber constructed from cylindrical glass tubing. A metal piston with a tight O-ring seal is fitted into the glass tubing. The ceiling of the chamber consists of a slightly concave surface of a 1% agarose gel cylinder attached to the metal piston, generating a completely hydrophilic surface for contact with the bubble. The temperature of the chamber was maintained at 37°C. We used the spreading technique described in Codd et al. (26), with slight modifications. This technique allowed us to examine the surface activity of very low volumes of surfactant at a relatively high concentration. Briefly the captive bubble chamber (1 mL volume) was filled with a buffered salt solution [0.9% NaCl, 10 mM N-2-hydroxyethylpiperazine-N ′-2-ethanesulfonic acid (HEPES), 2.5 mM CaCl2, pH 6.9], which contained 10%, by mass, sucrose. The sucrose was added to increase the density of the salt solution to approximately 1.04 g/mL, above that of the surfactant suspension (1.01 g/mL). Two microliters of 10 mg/mL from each sample was injected into the chamber near its agarose ceiling. The injected surfactant rests against the agarose ceiling at the top of the chamber. A bubble 6–7 mm in diameter was then created by moving the chamber down relatively to the stationary piston, and drawing air in by means of a small hole in the chamber base.
Calculation of surface tension area and volume.
The bubble was recorded continuously throughout the experiment on a video recorder (Sony EVO-9800A recorder (Sony of Canada, Toronto, Ontario, Canada) and Pulnix TM-7EX camera (Pulnix America Inc., Sunnyvale, CA, U.S.A.)). Surface tension, area, and volume were calculated from video images of the bubble height and diameter. Minimum surface tension was obtained when the bubble ceased to flatten and began to decrease in its width upon small compression steps.
Adsorption measurements.
Initially, a bubble was introduced into the chamber through a small inlet at the bottom of the chamber. The bubble moves up through the surfactant solution and comes to rest at the agarose surface. The moment the bubble comes to rest and assumes a Laplacian shape was considered to be time zero. Adsorption was assessed by measuring the decrease in surface tension over time.
Quasi-static cycling.
After adsorption to the equilibrium surface tension of ∼25 mN/m, the bottom inlet of the bubble chamber was sealed and the film at the bubble air-liquid interface was compressed stepwise, by reducing the bubble volume by 10% at each step and waiting for 10 s after each step. We conducted four quasi-static cycles with an intercycle delay of 2 min. The waiting periods at each step and the intercycle delays allowed the system to equilibrate. The quasi-static cycles were performed to establish the limits for the volume changes required for the maximum surface tension of approximately 30 mN/m and the minimum of less than 5 mN/m for dynamic cycling.
Dynamic cycling.
After the four quasi-static cycles, a waiting period of 2 min was observed and the bubbles were then cycled dynamically at 20 cycles/min by using the approximate volume limits established before. Compression was stopped at the time point when the bubble shape ceased to flatten and the minimum surface tension was achieved.
Lung histology.
At completion of the experiments, some lungs were prepared for histologic assessment after airway inflation (10 cm H2O) and fixation with formalin. Standard paraffin-blocking sectioning of the lung was used, as previously described (27). The lung slices were stained with hematoxylin and eosin and examined by light microscopy.
Statistical analysis.
Values are mean ± SE. Experimental and control data were compared by one- or two-way ANOVA. Multiple comparisons were done by the Tukey test utilizing the “Number Cruncher” Statistical Software (NCSS, Kaysville, UT, U.S.A.). Significance was accepted at p < 0.05.
RESULTS
Mechanical ventilation was associated with an increase in total respiratory system compliance (p < 0.01), both in absolute terms, and as a percent increase from time zero values (Fig. 1). These changes were statistically significant (p < 0.05) starting from the 10-min time points, for both the 10 and 40 mL/kg groups. Furthermore, compliance was greater in the animals ventilated with 40 mL/kg tidal volume compared with 10 mL/kg (Fig. 1).
The alveolar surfactant composition changed with mechanical ventilation. After 60 min of 10 mL/kg tidal volume ventilation, the total surfactant (Fig. 2A, P < 0.05) and LA fraction (Fig. 2B, p < 0.01) significantly increased, whereas the SA fraction decreased (Fig. 2C, p < 0.05).
A 40 mL/kg tidal volume resulted in a similar and significant increase in total surfactant (Fig. 3A, p < 0.05) after 60, but not 180 min ventilation. The LA fraction was significantly greater than the control values at 180 min of ventilation (Fig. 3B, p < 0.05) and the SA fraction was not altered by mechanical ventilation (Fig. 3C).
Significant changes in the surfactant surface-tension properties were observed after ventilation with a tidal volume of 40 mL/kg. The film adsorption at 1 s for the nonventilated rats was 34 ± 6 mN/m and not statistically different from the values measured in animals ventilated for 30 min and 60 min with a tidal volume of 40 mL/kg (26 ± 2, 36 ± 5 mN/m, respectively). In these animals, further film adsorption measurements at 1 and 5 min were similar to the 1-s values.
The minimum surface tension measured under dynamic cycling conditions was significantly higher in the animals ventilated with a tidal volume of 40 mL/kg for 60 min, as compared with the nonventilated values (Fig. 4). In keeping with the worsening surface-tension properties and decreased total surfactant, the lung dynamic compliance decreased past 60 min and at 180 min was no longer significantly different from time zero values (Fig. 5).
Minimum surface tension measured during dynamic cycling (20 cycles/min) in lung lavage material from nonventilated controls (n = 4), 30-min (n = 4), and 60-min (n = 4) ventilated animals with a tidal volume of 40 mL/kg. The minimum surface tensions were taken from the cycles numbers 1, 2, 9, and 10. **p < 0.01 by two-way ANOVA, as compared with control values.
Figure 6 shows representative lung histology of animals ventilated for 60 min with either 10 or 40 mL/kg tidal volumes. No significant gross changes in the lung parenchyma of ventilated animals were observed, as compared with controls.
DISCUSSION
In the newborn rat, mechanical ventilation with tidal volumes above the physiologic range induced a short-lived increase in total lung surfactant and improved total respiratory compliance. Such effect was observed when either 10 mL/kg, or a higher and potentially more injurious tidal volume (40 mL/kg PEEP = 0) was used. The lung surfactant biophysical properties were adversely affected by 40 mL/kg tidal volume ventilation and the total surfactant content at 180 min of ventilation was no longer different from the measured in the nonventilated animals.
In the lung, the epithelial type II cells secrete surfactant into the alveoli as lamellar bodies. These bodies unravel into multilaminar and lipid-protein tubular myelin-like structures containing SP-A, -B, and -C. These forms are referred to as large aggregates and can be separated from the lavage via high speed centrifugation (16). The surface tension–lowering properties of surfactant are related to this LA pool within the airspace. During respiration, LA are converted into smaller, vesicular lipid structures called SA that have poor surface tension-lowering properties (28, 29). Previous in vitro and in vivo studies have shown that phasic alveolar stretch induced by different tidal volumes is an important mediator of LA conversion. In addition, mechanical stretch induces expression of SP-C in fetal lung cells (30).
In this study, we have documented that the increase in alveolar surfactant pools induced by ventilation with 10 mL/kg and 40 mL/kg tidal volume is associated with an increase in the LA subfraction. Because LA represents the functionally active forms of alveolar surfactant, these changes likely explain the initial improvement in lung compliance observed in these animals.
In adult animals, the available data also suggest that alveolar distention results in surfactant release and/or secretion. For example, stretching of alveolar epithelial cells in vitro resulted in surfactant secretion (1), and, in adult rats, exercise (swimming) was associated with an increase in alveolar surfactant pools (2). Furthermore, 15 min of ventilation with high tidal volume caused release and/or secretion of surfactant (3), and in isolated perfused lungs given a single high tidal volume breath, surfactant pools were increased in rat lung lavage (31).
Other animal studies, however, have shown that, in spite of this stretch-induced increase in surfactant content, the isolated lung compliance after high tidal volume ventilation actually decreased (4). As shown in this study, this discrepancy is likely related to the fact that, although the total surfactant and its LA subfraction content initially increase, its surface tension–lowering properties are altered by ventilation with a high tidal volume. In fact, in the adult rat isolated lung preparation high tidal volume ventilation adversely affects pulmonary surfactant (32).
In this study, we chose to ventilate one group of animals with 10 mL/kg tidal volume, as previously reported in a clinical study of healthy term neonates (17). In addition, a doubling of the physiologic tidal volume is commonly used for recruitment maneuvers in ventilated neonates. Indeed, our protocol may well be applicable to the effects of recruitment maneuvers often used with mechanical ventilation. Whether recruitment maneuvers for mechanically ventilated neonates are of clinical benefit is debatable (33). In healthy neonates, recruitment seems to improve lung compliance (17), yet, lung recruitment at birth does not improve the response to surfactant in immature lambs, and may have an adverse effect on lung function and morphology (34).
Although we documented in the present study an initial increase in compliance and surfactant pools, mechanical ventilation of the newborn with a high tidal volume and PEEP = 0 for longer than 60 min alters the surfactant biophysical properties and likely results in lung injury. These changes can occur after a short exposure to mechanical ventilation. This is best illustrated by the recent evidence from Gajic et al. (35), where adult rats ventilated with a tidal volume of 40 mL/kg and PEEP = 0 for as little as 30 min showed significant lung parenchymal cell injury. The lung injury in the latter study (35), was only evident utilizing a cell membrane impermeable fluorescent marker and confocal microscopy. Thus, the normal appearance of the ventilated newborn lungs in this study does not exclude the possibility of significant injury at the cellular level.
The preterm lung is highly susceptible to injury during resuscitation or more chronic mechanical ventilation because the gas volumes per kilogram body weight of the lungs are small (36). Lung injury can be initiated in the immediate neonatal period if excessive tidal volumes are used during resuscitation and ventilation (33). Further evaluation of intermittent ventilation with a higher than physiologic tidal volume on the lung compliance and surfactant content of diseased and immature lungs is warranted. Such data will be of clinical relevance toward addressing the potential benefit of the use of lung recruitment maneuvers in newborns.
In summary, we demonstrated for the first time that total surfactant increases in healthy term newborn rats ventilated with a tidal volume higher than physiologic. This is associated with a significant but short-lived increase in total respiratory lung compliance. We speculate that the beneficial improvement in lung function in neonates after recruitment maneuvers with tidal volumes slightly higher than physiologic for a short duration is related to an increase in lung surfactant content.
Abbreviations
- ARDS:
-
acute respiratory distress syndrome
- LA:
-
large aggregate surfactant subfraction
- PEEP:
-
positive end-expiratory pressure
- SA:
-
small aggregate surfactant subfraction
- SP-A:
-
surfactant protein-A
- SP-B:
-
surfactant protein-B
- SP-C:
-
surfactant protein-C
References
Wirtz HR, Dobbs LG 1990 Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269.
Nicholas TE, Power JH, Barr HA 1982 Surfactant homeostasis in the rat lung during swimming exercise. J Appl Physiol 53: 1521–1528.
Nicholas TE, Barr HA 1983 The release of surfactant in rat lung by brief periods of hyperventilation. Respir Physiol 52: 69–83.
Veldhuizen RA, Welk B, Harbottle R, Hearn S, Nag K, Petersen N, Possmayer F 2002 Mechanical ventilation of isolated rat lungs changes the structure and biophysical properties of surfactant. J Appl Physiol 92: 1169–1175.
Veldhuizen RA, Ito Y, Marcou J, Yao LJ, McCaig L, Lewis JF 1997 Effects of lung injury on pulmonary surfactant aggregate conversion in vivo and in vitro. Am J Physiol 272: L872–L878.
Dreyfuss D, Saumon G 1998 Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323.
Parker JC, Hernandez LA, Peevy KJ 1993 Mechanisms of ventilator-induced lung injury. Crit Care Med 21: 131–143.
Kavanagh BP, Slutsky AS 1999 Ventilator-induced lung injury: more studies, more questions. Crit Care Med 27: 1669–1671.
Hickling KG, Henderson SJ, Jackson R 1990 Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 16: 372–377.
Hickling KG, Walsh J, Henderson S, Jackson R 1994 Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 22: 1568–1578.
Mariani G, Cifuentes J, Carlo WA 1999 Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 104: 1082–1088.
Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, Todd TR, Slutsky AS 1998 Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med 338: 355–361.
Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, Clementi E, Mancebo J, Factor P, Matamis D, Ranieri M, Blanch L, Rodi G, Mentec H, Dreyfuss D, Ferrer M, Brun-Buisson C, Tobin M, Lemaire F 1998 Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med 158: 1831–1838.
Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P Jr, Wiener CM, Teeter JG, Dodd-o JM, Almog Y, Piantadosi S 1999 Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 27: 1492–1498.
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR 1998 Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347–354.
The Acute Respiratory Distress Syndrome Network 2000 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308
Ratjen F, Zinman R, Stark AR, Leszczynski LE, Wohl ME 1989 Effect of changes in lung volume on respiratory system compliance in newborn infants. J Appl Physiol 67: 1192–1197.
Sanchez-Esteban J, Cicchiello LA, Wang Y, Tsai SW, Williams LK, Torday JS, Rubin LP 2001 Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol 91: 589–595.
Cotten M, Clark RH 2001 The science of neonatal high-frequency ventilation. Respir Care Clin N Am 7: 611–631.
Nakamura T, Malloy J, McCaig L, Yao LJ, Joseph M, Lewis J, Veldhuizen R 2001 Mechanical ventilation of isolated septic rat lungs: effects on surfactant and inflammatory cytokines. J Appl Physiol 91: 811–820.
Volgyesi GA, Tremblay LN, Webster P, Zamel N, Slutsky AS 2000 A new ventilator for monitoring lung mechanics in small animals. J Appl Physiol 89: 413–421.
Malloy JL, Veldhuizen RA, Lewis JF 2000 Effects of ventilation on the surfactant system in sepsis-induced lung injury. J Appl Physiol 88: 401–408.
Veldhuizen RA, Marcou J, Yao LJ, McCaig L, Ito Y, Lewis JF 1996 Alveolar surfactant aggregate conversion in ventilated normal and injured rabbits. Am J Physiol 270: L152–L158.
Duck-Chong CG 1979 A rapid and sensitive method for determining phospholipid phosphorus involving digestion with magnesium nitrate. Lipids 14: 492–497.
Palmer D, Schurch S, Belik J 2000 Effect of budesonide and salbutamol on surfactant properties. J Appl Physiol 89: 884–890.
Codd JR, Schurch S, Daniels CB, Orgeig S 2002 Torpor-associated fluctuations in surfactant activity in Gould's wattled bat. Biochim Biophys Acta 1580: 57–66.
Belik J, Davidge ST, Zhang W, Pan J, Greer JJ 2003 Airway smooth muscle changes in the nitrofen-induced congenital diaphragmatic hernia rat model. Pediatr Res 53: 737–743.
Veldhuizen RA, Hearn SA, Lewis JF, Possmayer F 1994 Surface-area cycling of different surfactant preparations: SP-A and SP- B are essential for large-aggregate integrity. Biochem J 300: 519–524.
Veldhuizen RA, Yao LJ, Lewis JF 1999 An examination of the different variables affecting surfactant aggregate conversion in vitro. Exp Lung Res 25: 127–141.
Nakamura T, Liu M, Mourgeon E, Slutsky A, Post M 2000 Mechanical strain and dexamethasone selectively increase surfactant protein C and tropoelastin gene expression. Am J Physiol 278: L974–L980.
Nicholas TE, Power JH, Barr HA 1982 The pulmonary consequences of a deep breath. Respir Physiol 49: 315–324.
Veldhuizen RA, McCaig LA, Akino T, Lewis JF 1995 Pulmonary surfactant sub-fractions in patients with the acute respiratory distress syndrome. Am J Respir Crit Care Med 152: 1867–1871.
Auten RL, Vozzelli M, Clark RH 2001 Volutrauma. What is it, and how do we avoid it? Clin Perinatol 28: 505–515.
Bjorklund LJ, Ingimarsson J, Curstedt T, Larsson A, Robertson B, Werner O 2001 Lung recruitment at birth does not improve lung function in immature lambs receiving surfactant. Acta Anaesthesiol Scand 45: 986–993.
Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD 2003 Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med 167: 1057–1063.
Jobe AH, Ikegami M 1998 Mechanisms initiating lung injury in the preterm. Early Hum Dev 53: 81–94.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the Canadian Institutes of Health and Research.
Rights and permissions
About this article
Cite this article
Martinez, F., Lewis, J., Copland, I. et al. Mechanical Ventilation Effect on Surfactant Content, Function, and Lung Compliance in the Newborn Rat. Pediatr Res 56, 19–25 (2004). https://doi.org/10.1203/01.PDR.0000128980.82797.29
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000128980.82797.29