Abstract
Trifunctional protein (TFP) plays a significant role in the mitochondrial β-oxidation of long-chain fatty acids. Its deficiency impairs the energy generating function of this pathway and causes hypoketotic hypoglycemia once hepatic glycogen stores are depleted. A Reye-like syndrome, cardiomyopathy, and sudden death may follow. The diagnosis is based on demonstration of significantly decreased enzyme activity of at least two of the three involved enzymes in fibroblasts. The possibility of prospective diagnosis of TFP deficiency by newborn screening using tandem mass spectrometry (MS/MS) has not been evaluated. We report the postmortem diagnosis of a male newborn, who suffered sudden death at 2 wk of age, and his younger sister, who died of cardiomyopathy complicated by acute heart failure at the age of 6 mo, after she had acquired a common viral infection. Blood spots from the original newborn screening cards were the only remaining material from the patients. Analysis by MS/MS revealed acylcarnitine profiles consistent with either TFP or long-chain 3-hydroxyacyl coenzyme A dehydrogenase (LCHAD) deficiency. To prove the diagnosis, the α- and β-subunit genes coding for TFP were examined. The patients were compound heterozygous for a 4-bp-deletion and an a→g missense mutation, both in the same exon 3 donor consensus splice site. This is the first report of the diagnosis of TFP deficiency using blood spots obtained for newborn screening and suggests that TFP deficiency may be detectable by prospective newborn screening using MS/MS.
Similar content being viewed by others
Main
Trifunctional protein (TFP) is an enzyme complex involved in the mitochondrial β-oxidation of fatty acids with chain lengths of C12 to C16. It is a hetero-octamer of 4 α- (McKusick #600890) and 4 β-subunits (McKusick #143450). The three enzymes are long-chain enoyl-CoA hydratase, long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), and long-chain 3-ketoacyl-CoA thiolase. The ketothiolase is encoded by the β-subunit gene, whereas the α-subunit gene codes for the other two enzymes. The first of the 4 steps of the β-oxidation cycle is catalyzed by an acyl-CoA dehydrogenase. The clinical presentation of TFP deficiency is similar to isolated LCHAD deficiency(1). Impaired degradation of fatty acids leads to a shortage of acetyl-CoA and to hypoketotic hypoglycemia, which is one of the key features during fasting. Further symptoms may include cardiomyopathy and arrhythmia, hepatomegaly with elevated aminotransferases and hepatic steatosis, progressive neuropathy, retinopathy, and myopathy. 3-Hydroxydicarboxylic acids with a chain length of 10 to 14 carbon atoms are elevated in urine from patients with TFP and LCHAD deficiencies. The diagnosis is confirmed by measuring the enzyme activities in fibroblasts and by DNA analysis (for review, seeRef. 2). Treatment consists of a low-fat, high-carbohydrate diet with medium-chain triglycerides as the main fat source.
A few patients have been described with a milder, late-onset variant of TFP deficiency(3,4). However, as patients may develop progressive cardiomyopathy early and die, prompt diagnosis and initiation of therapy are extremely important. Therefore, prospective diagnosis by newborn screening would be preferable. Tandem mass spectrometry (MS/MS) has evolved as one potential screening method, because it allows for the detection of abnormal acylcarnitines and amino acids in a single dried blood spot in a timely and cost-efficient manner(5,6). More than 20 inborn errors of metabolism are detectable using this technique(7,8), but its reliability to prospectively identify specific disorders has been documented for only a few genetic metabolic diseases, including medium-chain acyl-CoA dehydrogenase (MCAD) deficiency, one of the most common fatty acid β-oxidation disorders(9–12). Nevertheless, several states within the United States and some countries worldwide are currently either already using this method or are in the process of setting it up for newborn screening.
The genes encoding the α- and β-subunits of the trifunctional protein were identified in 1993, allowing for diagnosis of LCHAD and TFP deficiency by molecular genetic analysis(13). So far more than 25 mutations have been identified in the α-subunit, and 3 in the β-subunit (14–17; Strauss AW, unpublished data). One missense mutation (G1528C) was demonstrated as the most common in patients with isolated LCHAD deficiency(18–20).
We report the postmortem diagnosis of TFP deficiency in a sibship by acylcarnitine analysis in the blood spots from the original newborn screening cards and subsequent verification of the diagnosis by molecular genetic analysis from the same blood spots as the only remaining samples from these patients.
CASE REPORTS
Patient 1. After an uncomplicated term pregnancy, the male patient was born by vaginal delivery to healthy, nonconsanguineous parents. Birth weight was 2950 g (10th percentile). The perinatal course was unremarkable, and a blood specimen for newborn screening (PKU, galactosemia, MSUD, biotinidase deficiency, hypothyroidism) was obtained on his 5th day of life, the customary age for newborn screening in Germany. The infant was found unresponsive in his crib at 14 d old, and resuscitation allowed survival for only a few hours. Autopsy revealed a dilated cardiomyopathy and hepatic steatosis, but an underlying diagnosis was not established.
Patients 2. This female was two years younger than her brother, patient 1. She was also born after normal pregnancy and delivery. Blood for newborn screening was collected on her 5th day of life. She developed appropriately until 6 mo of age, at which time she manifested feeding problems, episodes of apnea, and lethargy. The infant was admitted to Reutlingen Childrens' Hospital in a comatose state. Laboratory values upon admission revealed hypoglycemia (11 mg/dL, normal 70-110 mg/dL), mild hyperammonemia (94 µmol/L, normal 5-50 µmol/L), elevation of transaminases (AST 95 U/L, normal 15-55 U/L; ALT 66 U/L, normal 5-45 U/L), creatine kinase (CK: 1206 U/L, normal <200 U/L; CK-MB: 50 U/L, normal <10 ng/mL), and LDH (569 U/L, normal 50-150 U/L). Intensive care treatment, including reversal of catabolism with intravenous glucose, lead to short lasting improvement, but the patient died of heart failure with dilated cardiomyopathy 2 days after admission. Urine organic acid analysis revealed elevated dicarboxylic and hydroxy-dicarboxylic acids. Fibroblasts were grown from a skin biopsy but enzymatic analysis was not possible due to bacterial contamination.
METHODS
Acylcarnitine analysis. Samples from the blood spots were prepared and analyzed as described previously(21,22). As internal standards, [2H3]-acetyl-, [2H3]-propionyl-, [2H3]-butyryl-, [2H3]-octanoyl-, and [2H3]-palmitoylcarnitine were added during sample preparation. The methylated samples were analyzed on a VG Quattro-1 triple quadrupole tandem mass spectrometer (Micromass Inc., Beverly, MA). The ion intensities of the analytes were compared with those of the appropriate internal standards by computer analysis using the NeoLynx® software (Micromass Ltd., Manchester, UK). The concentration of the different acylcarnitines was calculated from the ion intensity ratio except for analytes for which no reference standards were available. Because the patients' blood spots were of different ages at the time of analysis (31 mo: patient 1; 7 mo: patient 2), two different reference ranges for acylcarnitine concentrations or ion ratios were established to make up for the possible influence that long term storage of the blood spots may have had on the acylcarnitine concentrations. The blood spots from the patients and the healthy control population were obtained at the age of 5 d. The control populations' blood spots were analyzed 25 mo and 6 wk after collection, respectively, using the same methods and instrument as those described for the patient samples. Parental consent was obtained for release and analysis of the patients' newborn screening cards and all control blood spots were made anonymous by the screening laboratory before submission for analysis.
Molecular genetic analysis. Because only small amounts of DNA from the remaining blood spots were available, molecular analysis was first done for the patients' parents. After extraction of DNA from parental blood samples, single-strand conformation polymorphism (SSCP) analysis and DNA sequencing of all 20 TFP α-subunit exons were performed as described earlier(14). After mutation detection in both the father and the mother, exon-specific SSCP analysis and sequencing were performed using DNA from the remaining blood spot fragments from the newborn screening cards of the two patients. The Institutional Review Board of Washington University approved these analyses, for which parental consent was obtained.
RESULTS
Acylcarnitine analysis. The acylcarnitine profiles obtained from the newborn screening cards of the two patients and of two healthy controls are shown in Figure 1. The blood spots of the controls were analyzed 25 mo and 6 wk after collection from healthy 5-day-old newborns. The profiles of both patients were abnormal as compared with the control samples, although the differences were more obvious for patient 2. Her profile clearly showed elevations of long-chain acylcarnitines and long-chain hydroxy-acylcarnitines. In patient 1, the acylcarnitine profile did not appear significantly abnormal upon inspection. However, computer analysis revealed that the concentration of 3-hydroxy-palmitoylcarnitine (OH-C16), the typically elevated metabolite in LCHAD deficiency, was in fact significantly increased, as were some other long-chain acylcarnitines (Table 1). The peak for acetylcarnitine (C2) was decreased in comparison to that of his sister, which was in the low normal range relative to the healthy controls that were analyzed 6 wk after sample collection.
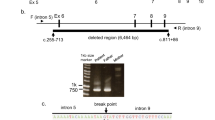
Acylcarnitine profiles of the patients' original newborn screening bloodspots and bloodspots of different ages from healthy newborns (control). Note differences in peak height of C2-, and long-chain acylcarnitines (C10 and longer). *Denotes internal standards (*2: [2H3]-acetylcarnitine; *3: [2H3]-propionylcarnitine; *4: [2H3]-butyrylcarnitine; *8: [2H3]-octanoylcarnitine; *16: [2H3]-palmitoylcarnitine).
Molecular genetic analysis. SSCP analysis of amplified DNA of all 20 TFP α-subunit exons using DNA from the blood spot of patient 2 revealed aberrant bands in exon 3, compared with the normal control (data not shown). Because of a limited supply of DNA from this source, further analysis was done with DNA isolated from the parents' blood. Direct sequencing of amplified maternal exon 3 DNA (Fig. 2) revealed heterozygosity for A and G at the +3 position of the donor splice consensus sequence. The paternal mutation was determined by sequence analysis of 12 subclones of his exon 3 amplified DNA. In five subclones, the normal exon 3-intron boundary (AGgtatcta) was altered to A-at-ta, an overall deletion of 4 bp, including the terminal nucleotide of the exon, the highly conserved GT at the first two positions of the donor consensus splice site, and C at position +5. Both mutations were confirmed by SSCP and sequence analysis of amplified exon 3 DNA from both children. We conclude that both children are compound heterozygotes for two different mutations in the same exon 3 donor consensus splice site.
Exon 3-intron boundary consensus donor splice site of a subclone from patient 1, his mother, and a normal control. The sequence of the patient's subclone reveals a deletion of overall 4 bp, for which his father is heterozygous (not shown). The maternal sequence demonstrates heterozygosity for a and g at the +3 position.
DISCUSSION
This is the first description of the diagnosis of TFP deficiency using blood spots from the newborn screening card by MS/MS. However, the diagnoses were not made in the setting of prospective newborn screening but postmortally on the basis of the patients' medical history. These cases underscore that the clinical manifestations of complete TFP and isolated LCHAD deficiencies are indistinguishable, and MS/MS analysis also appears unable to differentiate between these β-oxidation defects(23). Because treatment of both disorders, however, follows the same strategy, a preliminary diagnosis of a long-chain fatty acid oxidation defect by MS/MS is sufficient to justify initiation of therapy. Demonstration of the characteristic enzyme activity deficiencies in fibroblast cultures has been considered the gold standard for confirming the diagnosis. However, the identification of the genes encoding the α- and β-subunit of the trifunctional protein, which include LCHAD activity, has made a definite molecular diagnosis possible. Because only limited DNA from our patients was available after acylcarnitine analysis, combined molecular genetic analysis using DNA from parental blood and from the newborn screening blood spot was required to characterize the TFP α-subunit mutations in this sibship, for whom fibroblast cultures were not available. Both mutations alter the donor consensus splice site after exon 3. The maternal allele, A +3G, is identical to one of the mutations originally reported14 in a family with biochemically proven TFP deficiency that caused skipping of exon 3, a frame shift, and generation of a premature termination codon. The paternal mutation would also cause a frame shift, generating a premature termination codon and rapid α-subunit mRNA degradation(24). Because the β-subunit is proteolytically degraded rapidly in the absence of the α-subunit(17), it is likely that these mutations would cause complete TFP deficiency.
Mitochondrial fatty acid β-oxidation disorders have been recognized more frequently in recent years. However, the diagnosis of the deficiency of a specific β-oxidation enzyme is still often made during a postmortem work-up for sudden death in infancy(25,26). A prospective diagnosis for such a disorder is often based on the previous diagnosis in another family member or the occurrence of a pregnancy complication, such as acute fatty liver of pregnancy, in the mother (26,27; personal observations). Once symptomatic cardiomyopathy has developed, it may be impossible to stabilize or improve the condition of patients with disorders like LCHAD and TFP deficiency. Therefore, prospective diagnosis as early in life as possible is desirable. Newborn screening using MS/MS has already proven successful for MCAD deficiency, demonstrating an incidence of 1:17 706 in North Carolina and Pennsylvania(11). TFP and LCHAD deficiencies taken together might be as frequent as MCAD deficiency(28), but data on their detection by newborn screening using MS/MS have not been reported.
Compared with controls matched for patient age at sample collection and age of the sample itself, the acylcarnitine profiles of both patients are highly indicative for LCHAD or TFP deficiency. Upon inspection the acylcarnitine profile of patient 1 did not have strikingly abnormal 3-OH-palmitoylcarnitine peak which is typically seen in TFP or LCHAD deficiency. Quantification of the acylcarnitine signals relative to internal standard, however, demonstrate clearly abnormal elevations of hydroxylated long-chain acylcarnitines (Table 1). In addition, the near absent acetylcarnitine (C2) peak requires consideration. This finding may be indicative for secondary carnitine deficiency in the TFP deficient patient at the time the blood was drawn. Because import of long-chain fatty acids into the mitochondria is carnitine dependent, significant elevations of long-chain acylcarnitines may be absent if the patient is carnitine deficient. It may be hypothesized that the amount of specific acylcarnitines correlates with the availability of carnitine. This seems supported by the findings in patient 2. Already, inspection of her acylcarnitine profile (analyzed 7 mo after collection) revealed more pronounced abnormalities typically seen in TFP or LCHAD deficiencies, as well as a normal concentration for C2 (Table 1). Nevertheless, another likely cause for the low acetylcarnitine level in patient 1 is a storage effect due to the long interval of 31 mo until analysis of the blood spot. As we demonstrate in Table 1 for acetylcarnitine, the concentration of short-chain acylcarnitines decreases significantly after lengthy storage.
Although, carnitine dependency is not applicable to medium-chain fatty acids for crossing the mitochondrial membranes because they do not require the carnitine shuttle, MCAD metabolites are also not detectable when the patient suffers secondary carnitine deficiency(11). However, when considering that LCHAD deficiency may be as common as MCAD deficiency it is surprising that not as many LCHAD deficient patients are being identified by newborn screening using MS/MS. Therefore, screening for LCHAD and TFP deficiencies with this technique seems not as straightforward as that for MCAD deficiency. Because clinical and historical information on the patient is generally not available for newborn screening, as many data as possible must be acquired from the acylcarnitine profile itself. Because we cannot prove that the long term storage of our patients' blood spots is the only reason for the decreased acetylcarnitine levels, these cases suggest the C2 level as a possible aide in assessing acylcarnitine profiles. It appears prudent that newborn screening programs using MS/MS as a screening technique not only have the instruments flag elevated, but also abnormally low signals of acetylcarnitine. Furthermore, the question arises whether newborn screening laboratories using MS/MS for acylcarnitine analysis have set the threshold to flag hydroxylated long-chain acylcarnitines too high.
Tandem mass spectrometry is currently becoming an integral part of newborn screening programs worldwide. It is suggested that more than 20 disorders are detectable using this technique, but for newborn screening efficacy has so far been proven for only a few inborn errors of metabolism. The present cases demonstrate that the prospective diagnosis of TFP or LCHAD deficiency by MS/MS may be possible, but requires skilled and cautious interpretation of the results on the basis of carefully established age-matched reference ranges and adequate flagging parameters. Acylcarnitine analysis of more newborn screening cards of patients with LCHAD or TFP deficiencies is warranted to further evaluate the potential of MS/MS as a screening method for these disorders. Until this is accomplished, pediatricians and obstetricians need to be aware of the fact that the reliability of MS/MS to rule out inborn errors of metabolism has so far been shown to be indubitable only for a few such disorders.
Abbreviations
- TFP:
-
mitochondrial trifunctional protein deficiency
- MS/MS:
-
tandem mass spectrometry
- LCHAD:
-
long-chain 3-hydroxyacyl coenzyme A dehydrogenase
References
Grünewald S, Bakkeren J, Wanders RA, Wendel U 1997 Neonatal lethal mitochondrial trifunctional protein deficiency mimicking a respiratory chain defect. J Inherit Metab Dis 20: 835–836
Roe CR, Coates PM 1995 Mitochondrial fatty acid oxidation disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1501–1533
Miyajima H, Orii KE, Shindo Y, Hashimoto T, Shinka T, Kuhara T, Matsumoto I, Shimizu H, Kaneko E 1997 Mitochondrial trifunctional protein deficiency associated with recurrent myoglobinuria in adolescence. Neurology 49: 833–837
Schaefer J, Jackson S, Dick DJ, Turnbull DM 1996 Trifunctional enzyme deficiency: adult presentation of a usually fatal β-oxidation defect. Ann Neurol 40: 597–602
Cederbaum SD 1998 SIDS and disorders of fatty acid oxidation: where do we go from here?. J Pediatr 132: 913–914
Sweetman L 1996 Newborn screening by tandem mass spectrometry. Clin Chem 42: 345–346
Rashed MS, Bucknall MP, Little D, Awad A, Jacob M, Alamoudi M, Alwattar M, Ozand PT 1997 Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin Chem 43: 1129–1141
Ziadeh R, Hoffman EP, Finegold DM, Hoop RC, Brackett JC, Strauss AW, Naylor EW 1995 Medium chain acyl-CoA dehydrogenase deficiency in Pennsylvania: neonatal screening shows high incidence and unexpected mutations frequencies. Pediatr Res 37: 675–678
Chace DH, Hillman SL, Millington DS, Kahler SG, Adam BW, Levy HL 1996 Rapid diagnosis of homocystinuria and other hypermethioninemias from newborns' blood spots by tandem mass spectrometry. Clin Chem 42: 349–355
Chace DH, Hillman SL, Millington DS, Kahler SG, Roe CR, Naylor EW 1995 Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem 41: 62–68
Chace DH, Hillman SL, Van Hove JL, Naylor EW 1997 Rapid diagnosis of MCAD deficiency: quantitative analysis of octanoylcarnitine and other acylcarnitines in newborn blood spots by tandem mass spectrometry. Clin Chem 43: 2106–2113
Chace DH, Millington DS, Terada N, Kahler SG, Roe CR, Hofman LF 1993 Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin Chem 39: 66–71
Kamijo T, Aoyama T, Komiyama A, Hashimoto T 1993 Molecular cloning of the cDNAs for the subunits of rat mitochondrial fatty acid beta-oxidation multienzyme complex. J Biol Chem 268: 26452–26460
Brackett JC, Sims HF, Rinaldo P, Shapiro S, Powell CK, Bennett MJ, Strauss AW 1995 Two α subunit donor splice site mutations cause human trifunctional protein deficiency. J Clin Invest 95: 2076–2082
Orii KE, Aoyama T, Wakui K, Fukushima Y, Miyajima H, Yamaguchi S, Orii T, Kondo N, Hashimoto T 1997 Genomic and mutational analysis of the mitochondrial trifunctional protein beta-subunit (HADHB) gene in patients with trifunctional protein deficiency. Hum Mol Genet 6: 1215–1224
Sims HF, Brackett JC, Powell CK, Treem WR, Hale DE, Bennett MJ, Gibson B, Shapiro S, Strauss AW 1995 The molecular basis of pediatric long chain 3-hydroxyyacyl-CoA dehydrogenase deficiency associated with maternal acute fatty liver of pregnancy. Proc Natl Acad Sci USA 92: 841–845
Ushikubo S, Aoyama T, Kamijo T, Wanders RJA, Rinaldo P, Vockley J, Hashimoto T 1996 Molecular characterization of mitochondrial trifunctional protein deficiency: formation of the enzyme complex is important for stabilization of both - and -subunits. Am J Hum Genet 58: 979–988
Ding J-H, Yang B-Z, Nada MA, Roe CR 1996 Improved detection of the G1528C mutation in LCHAD deficiency. Biochem Mol Med 58: 46–51
IJlst L, Ruiter JPN, Hoovers JMN, Jakobs ME, Wanders RJA 1996 Common missense mutation G1528C in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: characterization and expression of the mutant protein, mutation analysis on genomic DNA and chromosomal localization of the mitochondrial trifunctional protein α subunit gene. J Clin Invest 98: 1028–1033
IJlst L, Wanders RJA, Ushikubo S, Kamijo T, Hashimoto T 1994 Molecular basis of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of the major disease-causing mutation in the α-subunit of the mitochondrial trifunctional protein. Biochim Biophys Acta 1215: 347–350
Millington DS, Kodo N, Terada N, Roe D, Chace DH 1991 The analysis of diagnostic markers of genetic disorders in human blood and urine using tandem mass spectrometry with liquid secondary ion mass spectrometry. Int J Mass Spectrom Ion Proc 111: 211–228
Millington DS, Norwood DL, Kodo N, Roe CR, Inoue F 1989 Application of fast atom bombardment with tandem mass spectrometry and liquid chromatography/mass spectrometry to the analysis of acylcarnitines in human urine, blood, and tissue. Anal Biochem 180: 331–339
Shen J-J, Matern D, Millington DS, Hillman SL, Feezor M, Bennett MJ, Qumsiyeh M, Kahler SG, Chen Y-T, Van Hove JLK 1998 Acylcarnitines produced in vitro by cultured fibroblasts of patients with long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency and other disorders of fatty acid oxidation. Annual SIMD Meeting, Asilomar, (abstr)
Perlick HA, Medghalchi SM, Spencer FA, Kendzior RJ, Dietz HC 1996 Mammalian orthologues of a yeast regulator of nonsense transcript stability. Proc Natl Acad Sci USA 93: 10928–10932
Rashed MS, Ozand PT, Bennett MJ, Barnard JJ, Govindaraju DR, Rinaldo P 1995 Inborn errors of metabolism diagnosed in sudden death cases by acylcarnitine analysis of postmortem bile. Clin Chem 41: 1109–1114
Tyni T, Palotie A, Viinikka L, Valanne L, Salo MK, von Döbeln U, Jackson S, Wanders R, Venizelos N, Pihko H 1997 Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency with the G1528C mutation: clinical presentation of thirteen patients. J Pediatr 130: 67–76
Tyni T, Ekholm E, Pihko H 1998 Pregnancy complications are frequent in long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Am J Obstet Gynecol 178: 603–608
Roe CR 1997 Mitochondrial fatty acid oxidation disorders: chapter 45 update. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, CD-ROM
Acknowledgements
The authors thank Y.-T. Chen and Robert D. Stevens (Durham, NC) for stimulating discussion and advice, Beverly Gibson (St. Louis, MO) for expert technical assistance in molecular genetic analysis, and Andreas Schulze (Heidelberg, Germany) for provision of age-matched control blood spots.
Author information
Authors and Affiliations
Additional information
This work was partially supported by the Deutsche Forschungsgemeinschaft (MA 1964/1-1) (to Dietrich Matern).
Rights and permissions
About this article
Cite this article
Matern, D., Strauss, A., Hillman, S. et al. Diagnosis of Mitochondrial Trifunctional Protein Deficiency in a Blood Spot from the Newborn Screening Card by Tandem Mass Spectrometry and DNA Analysis. Pediatr Res 46, 45–49 (1999). https://doi.org/10.1203/00006450-199907000-00008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199907000-00008