Abstract
During weaning the infant has a high iron requirement, and highly available dietary iron is needed to ensure optimal iron status. Muscle tissue has been identified as an enhancer of nonheme iron absorption in adults, although the influence of meat on nonheme iron absorption in infants has not been previously reported. The effect of the addition of 25 g of meat (lean beef) on nonheme iron absorption from a home-prepared vegetable purée meal (80 g of vegetables) was investigated in infants in the present study. The meals did not differ in their contents of other known enhancers or inhibitors of nonheme iron absorption. Incorporation of stable isotopes of iron (57Fe and58 Fe) into red blood cells 14 d after intake was used to measure iron absorption, using a cross-over design in eight healthy infants 43-49 wk of age. Nonheme iron absorption was significantly increased (p = 0.002) from the vegetable purée with added meat (geometric mean 15.0%) compared with the puréed vegetables (geometric mean 9.9%). These results thus suggest that meat is also an enhancer of nonheme iron absorption in infants and that nonheme iron absorption from weaning foods can be increased by the addition of meat.
Similar content being viewed by others
Main
Iron deficiency is the most common nutritional deficiency in industrialized countries. Its prevalence is particularly high in late infancy when iron stores are depleted, growth velocity is high, and iron intake is usually low(1). Iron intake can be low during the weaning period due to the gradual increase in semisolid foods, in particular if home-prepared nonironfortified weaning foods are used. The consequences of iron deficiency during infancy can be severe and may include impairment of mental and psychomotor development(2) as well as a negative impact on cell-mediated immune function(3). The prevention of iron deficiency during late infancy should therefore be given high priority in public health promotion. Research to identify dietary factors that influence iron absorption from infant foods to optimize iron nutrition during infancy is an important part of this objective(4). Although iron absorption and dietary factors influencing iron absorption have been relatively well documented in adults, only limited information is available on iron absorption in infants. Infant foods have often been evaluated for iron absorption in studies with adults, although the validity of adults as a model for iron absorption in infants has not been demonstrated. There are physiologic reasons why infants may not absorb dietary iron similarly to adults. The gastric pH in infants is relatively high (around 5) compared with adults, and the secretion of several proteolytical enzymes is lower early in life(5). It is also possible that receptor-mediated processes of iron absorption only function only at a young age(6).
The lack of data on iron absorption in infants is primarily due to methodologic problems associated with studies of trace element absorption. Ethical considerations have limited the number of studies using radioisotopes in infants. Furthermore, the chemical balance technique has serious limitations when studying elements with a low fractional absorption as is the case for iron(7). Recently, stable isotope techniques to study iron absorption have been developed(8, 9). These techniques are based on the determination of stable isotope incorporation into erythrocytes 14 d after intake as a estimate for iron absorption. By using a double isotope technique, iron absorption can be measured from two different test meals administered on consecutive days and factors influencing iron absorption can be evaluated based on paired comparisons(9). The large interindividual variation in iron absorption in infants can thus be overcome.
Adult studies using radioisotopes have demonstrated that muscle tissue(meat, fish, and poultry) is a major enhancer of nonheme iron absorption(10–13). The enhancing effect has not been demonstrated in infants, although beneficial effects on iron status of meat containing diets have been reported(14, 15). We have recently conducted an intervention study in which infants were randomized to a low (10 g of meat/d) or a high meat diet (27 g of meat/d) during the period from 8 to 10 mo of age(16). The high meat diet had a significant positive effect on Hb levels. There were only minor differences in total iron intake between the two groups, suggesting that the high meat content improved iron absorption.
The aim of the present study was to evaluate the influence of muscle tissue(lean beef) on nonheme iron absorption from a home-prepared weaning meal based on puréed vegetables in infants. Iron absorption was measured by a double stable isotope technique.
METHODS
Subjects. Seven- to eight-month-old infants selected at random from the central national register were sought to participate in the study. The parents of three girls and five boys, all healthy, agreed to participate. The mean age at the time of the study was 45.5 wk (range 43-49), and mean birth weight was 3671 g (range 2471-4780). The parents were fully informed about the aims and procedures of the study both orally and in writing, and informed consent was obtained. The protocol was approved by the Ethics Committee for Copenhagen and Frederiksberg.
Stable isotope labels. Elemental iron, isotopically enriched in57 Fe (95.4% 57Fe) and 58Fe (91.7% 58Fe), respectively, was purchased from Isotec (France) and dissolved in 0.1 M H2SO4 to obtain isotopically labeled FeSO4 solutions. The isotopic composition of the iron in solution was determined by negative thermal ionization mass spectrometry using FeF4- molecular ions(17) and a magnetic sector field instrument (MAT 262, Finnigan MAT, Germany). Iron concentrations of the labeled solutions were determined against a diluted commercially available iron standard(Titrisol®, Merck, Germany) by isotope dilution mass spectrometry. The isotopically labeled solutions were diluted by mass to an appropriate concentration for preparation of the isotope doses.
The calculation of the isotope doses to be administered was based on the estimated total amount of circulating iron in the infants and the expected range of fractional iron absorption from the test meals. The total dose of added isotopes was divided into two equal parts, each added to a test meal, in order not to increase the total iron content excessively. The total nonheme iron content was kept constant between test meals by the addition of 345 μg of iron (test meal M) and 442 μg of iron (test meal V), respectively. The iron isotope doses were prepared by weighing the corresponding amounts of labeled solutions into Teflon containers, which were flushed with argon to keep the iron in the +II oxidation state.
Test meals. Test meals consisted of a vegetable purée and a vegetable purée to which lean beef was added(Table 1). The amount of vegetables present in the two test meals was identical. Energy density was kept at the same level by substituting energy from meat with energy from corn oil and corn starch in the vegetable purée. The test meals were prepared in bulk and filled into plastic boxes each containing 125 g and stored at -18 °C until used. Before administration, the meals were thawed and heated to 40 °C in a microwave oven. The prepared 57Fe isotope doses were added to the vegetable purée with added meat, whereas the 58Fe isotope doses were added to the vegetable purée without meat. The added stable isotope solutions were carefully mixed with the test meals to ensure a homogenously labeled test meal. After addition of the iron isotopic labels, the total nonheme iron content of both test meals was identical (see Table 1).
Analysis of test meals. Portions of freeze-dried purées were analyzed in triplicate for total iron and nonheme iron as well as for iron absorption inhibitors, i.e. calcium, phytic acid, and polyphenols. In addition, ascorbic acid was quantified, and the protein and energy contents of the purées were determined. For total iron and calcium analysis, aliquots of freeze-dried test meals were mineralized by microwave digestion (MLS 1200, MLS GmbH, Germany) using a mixture of HNO3 and H2O2. Iron and calcium in the mineralized samples were quantified by flame atomic absorption spectrometry (SpectrAA 400, Varian, Australia) by standard addition technique to minimize matrix effects. Nonheme iron was determined by UV-VIS spectrophotometry (Uvikon 940, Kontron, Germany) using the method described by Rhee and Ziprins(18). For complete recovery of intracellularly bound nonheme iron, the freeze-dried purées were enzymatically digested before analysis using a pectolytic enzyme preparation (Pectinex Ultra SP-L, Novo Nordisk Ferment Ltd., Switzerland). Heme iron was calculated as the difference between total and nonheme iron. Within all iron and calcium analysis the guidelines of inorganic trace analysis were strictly followed to minimize contamination risks(19, 20).
The phytic acid content of the test meals was determined colorimetrically as inorganic phosphorus according to the method by Makower(21), which was modified by using cerium sulfate instead of iron ferric chloride for a more selective phytic acid precipitation. The total polyphenol content of the test meals was ascertained using Folin-Ciocalteu reagent according to Celeste et al.(22). Ascorbic acid was measured in test meals which were treated in identical fashion to the labeled test meals, i.e. stored frozen for the same period of time, thawed in the same manner and heated to the same temperature. Ascorbic acid was extracted with an aqueous solution of meta-phosphoric acid (wt/vol 6%) and Na2EDTA (wt/vol 0.3%) (National Food Agency of Denmark, Denmark, unpublished method) and quantified by HPLC(Waters 6000A pump, Waters Wisp 710 B injector, Waters, Milford, MA) using a reversed phase column (Merck Lichrosorb RP-18, 5 μm, 250 × 4.6 mm) and photometric detection at 247 nm (Jasco 870-UV, Jasco, Japan). Nitrogen content was determined by N2 combustion (Dumas Method, NA 1500 Carlo Erba, Fisons Instruments, Italy); protein content was calculated from total nitrogen content using the conversion factor 6.25 g of protein/g of nitrogen. The chemical energy value was analyzed by combustion calorimetry (calorimeter system C 4000, IKA, Germany).
Study design. One labeled test meal was fed on 4 consecutive d. The infants started with either of the two test meals (V or M) followed by administrations in the order M-V-M (four infants) or V-M-V (four infants). The labeled test meals were given at lunchtime under close supervision by the investigators at the department. Bowls, spoons, and bibs were preweighed to estimate test meal losses during and after administration. The infants were offered a drink of ultrapure water together with the labeled test meal. No food or drink was allowed 3 h before and 3 h after intake of each test meal.
Blood samples. Venous blood samples (3 mL, EDTA treated; Venoject II, Therumo Europe, Belgium) were drawn the day before the first labeled test meal was administered and 14 d after intake of the last labeled test meal. Whole blood was used to determine Hb concentration and iron isotope ratios, whereas plasma samples were obtained for analysis of PF. Hb was measured directly on the day of sampling, whereas whole blood and plasma samples were stored at -80 °C for the remaining analyses, which were made within the next 6 mo. All analyses were performed in duplicate, and mean values were used in the statistical analysis. Hb was determined by the cyanomethemoglobin method on a Cobas Minos-ST® (Hoffmann-La Roche, Nutley, NJ). PF was determined by using a commercial enzyme immunoassay kit.(NovaPath™ ferritin kit, Bio-Rad, Italy, calibrated against WHO-1st IS 80/602). Body weight was measured to the nearest 1 g with an electronic scale(Sartorius IP 65, Sartorius, Germany) on the same days as the blood sampling.
Analysis of iron isotope ratios in blood samples. Within all experimental work involving the handling of isotopically enriched blood samples the guidelines for trace element analysis were strictly followed including additional purification of the commercially available chemicals and reagents as well as acid-washing of all used containers. All sample handling was done under clean laboratory conditions to reduce the risk of sample contamination during analysis. Each isotopically enriched blood sample was analyzed in duplicate under chemical blank monitoring.
Whole blood samples were thawed and 0.5 mL were transferred to a Teflon vessel. The blood samples were mineralized by microwave digestion (MLS 1200) using a mixture of HNO3 and H2O2. Iron was separated from the mineralized matrix by anion-exchange chromatography after a solvent/solvent extraction step into diethyl ether for additional purification(9, 23).
The iron isotopic composition of blood samples was determined according to the recently developed technique by Walczyk(17). Isotopic analysis of the iron separated from the blood sample was performed by thermal ionization mass spectrometry equipped with a magnetic sector field mass and a multicollector system for simultaneous ion beam detection (MAT 262). The samples ware loaded onto a BaF2-coated rhenium filament of a double-filament ion source together with AgF to promote the formation of negatively charged FeF4- molecular ions. Because of the high enrichment of the isotopically enriched labels and the low amounts of label incorporated into the red blood cells, the 54Fe/56Fe isotope ratio in the blood remains unchanged within the reproducibility of the isotopic analysis after red blood cell incorporation of the labels. Therefore, it was possible to normalize the acquired data for the natural isotope ratio54 Fe/56Fe = 0.06370(24) to correct for mass-dependent isotopic fractionation effects. The normalized iron isotope ratios of blood samples taken before test meal administration were identical to the natural iron isotope ratio(24).
Calculation of the fractional iron absorption. Based on the shift of the iron isotope ratios in the blood samples and the amount of iron circulating in the body, the amounts of 57Fe label and 58Fe label present in the blood of the subjects 14 d after test meal administrations were calculated. Calculations followed the principles of isotope dilution and considered that both iron labels were not monoisotopic(25). The calculation of circulating iron was based on estimated blood volume and the Hb concentration(9). For calculations of fractional absorption, 90% incorporation of the absorbed iron into red blood cells was assumed(26).
Statistical analysis. Paired t test was used for comparison of fractional iron absorption from the two test meals. Iron absorption values were logarithmically transformed before analysis and reconverted to antilogarithms to recover original units(27). The correlations between age, weight, hematologic parameters, and iron absorption were analyzed using Pearson correlation coefficients. All analyses were performed using the SAS Statistical Software(28).
RESULTS
Composition of the test meals and the contents of energy, protein, iron, calcium, polyphenols, phytic acid, and ascorbic acid in the two test meals are given in Table 1. The mean intake (±1 SD) of the test meals was 118.7 g (±16.0) corresponding to 92.0%. Mean intake(±1 SD) of ultrapure water was 30.5 g (±18.8). Individual data on infant characteristics at the time of the first blood sampling and nonheme iron absorption are given in Table 2. One infant was anemic (Hb < 105 g/L) and three infants had iron deficiency (PF < 10μg/L) at the time of the first blood sampling. None of the infants had iron deficiency anemia, i.e. Hb < 105 g/L and PF < 10 μg/L(29). The infant with anemia was treated with iron supplementation after the study.
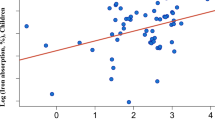
Nonheme iron absorption was significantly higher (p = 0.002) from the test meal containing meat. The geometric mean (range) was 15.0%(6.8-22.0), compared with 9.9% (4.8-13.7) from the vegetable purée. No statistically significant correlations were observed between age, weight, Hb, PF, and nonheme iron absorption.
DISCUSSION
The present study demonstrated that nonheme iron absorption in infants was significantly higher from a vegetable purée containing meat than from a vegetable purée without added meat. Apart from the added meat, the purées differed only in the content of corn oil, corn starch, and water. None of these ingredients is known to have any impact on iron absorption. There were no major differences between the purées in the content of the known inhibitors of nonheme iron absorption calcium(30, 31), polyphenols(32, 33), phytic acid(33, 34), or the well known enhancer, ascorbic acid(34, 35). Thus, there is good evidence that the increased nonheme iron absorption could be attributed to the added meat.
To our knowledge, only one previous study has reported information on nonheme iron absorption in infants from a weaning food containing meat(8). The authors concluded that nonheme iron absorption does not seem to be influenced by the presence of meat. The data from this previous study, however, are difficult to interpret because no direct comparison was made in the same infant consuming vegetables only.
In the present study, the mean fractional nonheme iron absorption increased 1.5-fold when meat was incorporated into a vegetable meal. This is a more modest enhancing effect than has been observed in adults. However, it would appear that, in the earlier adult studies, the meat was added to more inhibitory meals. For example, a review of several iron absorption studies in adults(36) reported that nonheme iron absorption was increased on average 2.6-fold when meat was added to a very inhibitory vegetable meal. The geometric mean fractional iron absorption from the vegetable meal with meat added was 15% in the present study, which is higher than in any of the adult studies reviewed by Lynch et al.(36).
Many attempts to isolate the “meat factor” responsible for enhancing nonheme iron absorption have been made, i.e. water-soluble extracts(12) and dilute acid-soluble extracts(37) of muscle tissue, divalent amino acids(38), cysteine-containing residues(39–41), actin, and myosin(13). Although it now seems likely that the “meat factor” is related to the cysteine-containing peptides, it is possible that more than one factor is involved. Zhang et al.(42) recently suggested the hypothesis that several factors act in synergism.
Of the nonheme iron in the vegetable purée with added meat, a mean absorption of 15% (134 μg) (nonheme iron from vegetables, meat, and stable isotope label) was found in the present study. If it is assumed that 25%(43–45) of the heme iron in meat was absorbed in addition, the added meat contributed an additional 103 μg of absorbed heme iron per test meal. The total quantity of absorbed iron from the vegetable purée with added meat was thus 237 μg. From the vegetable purée, a mean absorption of 9.9% or 88.7 μg of nonheme iron was found (nonheme iron from the vegetables and stable isotope label). The addition of 25 g of meat to 100 g of vegetable purée thus increased the total quantity of absorbed iron 2.7-fold. The total amount of iron absorbed(237 μg) from the vegetable purée with meat corresponds to one third of the estimated daily requirement of absorbed iron in 4-12-mo infants (0.75 mg/d)(45) compared with only 12% from the purée without added meat. Thus, vegetables served together with meat can be a good source of iron in the weanling's diet. The mean meat intake from the test meals was approximately 25 g, which is twice the average daily intake found in an observational study of infants from the same area(46). We have recently conducted an intervention study where infants consumed a diet containing 27 g of meat daily during the period from 8 to 10 mo of age(16). Thus, the amounts of meat in the test meals in this study are relatively high but realistic for this age group.
The validity of the extrinsic labeling technique for studies of nonheme iron absorption has been demonstrated with the use of radioisotopes(47, 48). With few exceptions, e.g. iron in milk(49) and poorly soluble forms of iron fortification compounds(50), added isotopes and nonheme iron in a meal forms a common pool. Thus erythrocyte incorporation of stable isotopes can be assumed to reflect absorption of nonheme iron from the test meals in this study. However, in contrast to radioisotopes, stable isotopes cannot be regarded as “true tracers” for the absorption of food nonheme iron as the quantities of stable isotopes added to the test meals, in particular in studies based on the incorporation of iron isotopes into erythrocytes, are not negligible(7). This is of less concern when iron absorption from iron-fortified foods such as infant formulas and infant cereals are studied(34, 51). However, when iron absorption is measured from foods that are not normally iron-fortified such as human milk(52), or homemade weaning foods as in the present study, the added amount of iron in the form of stable isotope has to be carefully evaluated. The dose of stable isotopes added to the test meals depends on several considerations, including analytical precision of the stable isotope ratio measurements, the study design, and the expected iron absorption(9). In this study, the total nonheme iron content was equilibrated in the test meals by addition of 57Fe and 58Fe. As a result, the iron added in the form of stable isotopes increased the iron contents of the test meals by 97%(vegetables) and 63% (vegetables and meat), respectively. A decreased fractional iron absorption has been demonstrated in adults with increasing iron doses(53), although no data are available on the effect of iron load on fractional iron absorption in infants. Therefore, the results from the present study could be expected to underestimate the“true” fractional iron absorption from the test meals containing only native iron. However, because the primary aim of the study was to investigate the magnitude of the influence of added meat on nonheme iron absorption from a weaning food containing equal amounts of nonheme iron in infants, the increased iron content is not expected to have any major impact on the interpretation of the results.
In conclusion, the present study shows for the first time that meat is an enhancer of nonheme iron absorption in infants. The addition of meat to vegetable-based weaning foods in industrialized countries can thus be used as a method for increasing iron supply to infants. In developing countries, small amounts of other animal muscle proteins such as dried fish might, if the enhancing effect on nonheme iron absorption is also confirmed in infants, provide a similar nutrition benefit.
Abbreviations
- PF:
-
plasma ferritin
References
Dallman PR, Siimes MA, Stekel A 1980 Iron deficiency in infancy and childhood. Am J Clin Nutr 33: 86–118.
Walter T 1992 Early and long-term effect of iron deficiency anemia on child development. In: Fomon SJ, Zlotkin SH (eds) Nutritional Anemias. Nestlé Nutrition Workshop Series, Vol 30. Raven Press, New York, pp 81–90.
Dallman PR 1987 Iron deficiency and the immune response. Am J Clin Nutr 46: 329–334.
Stekel A 1984 Prevention of iron deficiency. In: Stekel A(ed) Iron Nutrition in Infancy and Childhood. Nestlé Nutrition Workshop Series, Vol 4. Raven press, New York, pp 179–194.
Lebenthal E, Lee PC, Heitlinger LA 1983 Impact of development of the gastrointestinal tract on infant feeding. J Pediatr 102: 1–9.
Davidson LA, Lönnerdal B 1988 Specific binding of lactoferrin to brush-border membrane: ontogeny and effect of glycan chain. Am J Physiol 254:G580–G585.
Davidsson L 1994 The use of stable isotope techniques to study minerals and trace elements in infants. Monatschr Kinderheilkd 142:S20–S25.
Fomon SJ, Ziegler EE, Rogers RR, Nelson SE, Edwards BB, Guy DG, Erve JC, Janghorbani M 1989 Iron absorption from infant foods. Pediatr Res 26: 250–254.
Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF 1994 A double stable isotope technique for measuring iron absorption in infants. Br J Nutr 71: 411–424.
Layrisse M, Martinez-Torres C, Roche M 1968 Effect of interaction of various foods on iron absorption. Am J Clin Nutr 21: 1175–1183.
Cook JD, Monsen ER 1976 Food iron absorption in human subjects. III. Comparison of the effect of animal proteins on nonheme iron absorption. Am J Clin Nutr 29: 859–867.
Björn-Rasmussen E, Hallberg L 1979 Effect of animal proteins on the absorption of food iron in man. Nutr Metab 23: 192–202.
Hurrell RF, Lynch SR, Trinidad TP, Dassenko SA, Cook JD 1988 Iron absorption in humans: bovine serum albumin compared with beef muscle and egg white. Am J Clin Nutr 47: 102–107.
Haschke F, Pietschnig B, Vanura H, Heil M, Steffan I, Hobiger G, Schuster E, Camaya Z 1988 Iron intake and iron nutritional status of infants fed iron-fortified beikost with meat. Am J Clin Nutr 47: 108–112.
Mira M, Alperstein G, Karr M, Ranmuthugala G, Causer J, Niec A, Lilburne A-M 1996 Haem iron intake in 12 -36 month old children depleted in iron: case-control study. BMJ 312: 881–883.
Engelmann MDM, Sandström B, Michaelsen KF 1997 Meat intake and iron status in late infancy: an intervention study. J Pediatr Gastroenterol Nutr (in press)
Walczyk T 1997 Iron isotope ratio measurements by negative thermal ionisation mass spectrometry using FeF4- ions. Int J Mass Spectrom Ion Proc 161: 217–227.
Rhee KS, Ziprin YA 1987 Modification of the Schricker nonheme iron method to minimize pigment effects for red meats. J Food Sci 52: 1174–1176.
Zief M, Mitchell JW 1976 Contamination Control in Trace Element Analysis. Wiley, New York, pp 12–252.
Howard AG, Statham JW 1993 Inorganic Trace Analysis-Philosophy and Practice. Wiley, Chichester, pp 13–177.
Makower RU 1970 Extraction and determination of phytic acid in beans (Phaseolus vulgaris). Cereal Chem 47: 288–296.
Celeste M, Tomás C, Cladera A, Estela JM, Cerdá V 1992 Enhanced automatic flow-injection determination of the total polyphenol index of wines using Folin-Ciocalteu reagent. Anal Chim Acta 269: 21–28.
Beer B, Heumann KG 1993 Isotope dilution mass spectrometry of microelectronically relevant heavy metal traces in high-purity cobalt. Fresenius J Anal Chem 347: 351–355.
Taylor PDP, Maeck R, Biévre PD 1992 Determination of the absolute isotopic composition and atomic weight of a reference sample of natural iron. Int J Mass Spectrom Ion Proc 121: 111–125.
Walczyk T, Davidsson L, Zavalenta N, Hurrell RF 1997 Stable isotope labels as a tool to determine iron absorption by Peruvian school children from a breakfast meal. Fresenius J Anal Chem 359: 445–449.
Rios E, Hunter RE, Cook JD, Smith NJ, Finch CA 1975 The absorption of iron as supplements in infant cereal and infant formulas. Pediatrics 55: 686–693.
Cook JD, Layrisse M, Finch CA 1969 The measurement of iron absorption. Blood 33: 421–429.
SAS/STAT® Users Guide 1989 Version 6, 4th Ed, Vols 1 and 2. SAS Institute Inc., Cary, NC, pp 1-943 1–846.
Siimes MA, Salmenperä L, Perheentupa J 1984 Exclusive breast-feeding for 9 months: risk of iron deficiency. J Pediatr 104: 196–199.
Monsen ER, Cook JD 1976 Food iron absorption in human subjects. IV. The effects of calcium and phosphate salts on the absorption of nonheme iron. Am J Clin Nutr 29: 1142–1148.
Hallberg L, Rossander-Hultén L, Brune M, Gleerup A 1992 Bioavailability in man of iron in human milk and cow's milk in relation to their calcium contents. Pediatr Res 31: 524–527.
Tuntawiroon M, Sritongkul N, Brune M, Rossander-Hultén L, Pleehachinda R, Suwanik R, Hallberg L 1991 Dose-dependent inhibitory effect of phenolic compounds in foods on nonheme-iron absorption in men. Am J Clin Nutr 53: 554–557.
Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR, Mills W, Charlton RW 1983 The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr 49: 331–342.
Davidsson L, Galan P, Kastenmayer P, Cherouvrier F, Juilleret M-A, Hercberg S, Hurrell R 1994 Iron bioavailability studied in infants: the influence of phytic acid and ascorbic acid in infant formulas based on soy isolate. Pediatr Res 36: 816–822.
Hallberg L, Brune M, Rossander L 1986 Effect of ascorbic acid on iron absorption from different types of meals. Hum Nutr Appl Nutr 40A: 97–113.
Lynch SR, Hurrell RF, Dassenko SA, Cook JD 1989 The effect of dietary proteins on iron bioavailability in man. In: Dintzis FR, Laszlo JA (eds) Mineral Absorption in the Monogastric GI Tract. Plenum Press, New York, pp 117–132.
Slatkavitz CA, Clydesdale FM 1988 Solubility of inorganic iron as affected by proteolytic digestion. Am J Clin Nutr 47: 487–495.
Christensen JM, Ghannam M, Ayres JW 1984 Effects of divalent amino acids on iron absorption. J Pharm Sci 73: 1245–1248.
Layrisse M, Martinéz-Torres C, Leets I, Taylor P, Ramírez J 1984 Effect of histidine, cysteine, glutathione or beef on iron absorption in humans. J Nutr 114: 217–223.
Martinez-Torres C, Romano E, Layrisse M 1981 Effect of cysteine on iron absorption in man. Am J Clin Nutr 34: 322–327.
Taylor PG, Martinez-Torres C, Romano EL, Layrisse M 1986 The effect of cysteine-containing peptides released during meat digestion on iron absorption in humans. Am J Clin Nutr 43: 68–71.
Zhang D, Carpenter CE, Mahoney AW 1990 A mechanistic hypothesis for meat enhancement of nonheme iron absorption: stimulation of gastric secretions and iron chelation. Nutr Res 10: 929–935.
Hallberg L, Björn-Rasmussen E, Howard L, Rossander L 1979 Dietary heme iron absorption. A discussion of possible mechanisms for the absorption-promoting effect of meat and for the regulation of iron absorption. Scand J Gastroenterol 14: 769–779.
Bezwoda WR, Bothwell TH, Charlton RW, Torrance JD, MacPhail AP, Derman DP, Mayet F 1983 The relative dietary importance of haem and non-haem iron. S Afr Med J 64: 552–556.
Fomon SJ 1993 Iron. In: Fomon SJ (ed) Nutrition of Normal Infants. Mosby-Year Book, St. Louis, pp 239–260.
Michaelsen KF, Milman N, Samuelson G 1995 A longitudinal study of iron status in healthy Danish infants: effects of early iron status, growth velocity and dietary factors. Acta Paediatr 84: 1035–1044.
Cook JD, Layrisse M, Martinez-Torres C, Walker R, Monsen E, Finch CA 1972 Food iron absorption measured by an extrinsic tag. J Clin Invest 51: 805–815.
Björn-Rasmussen E, Hallberg L, Walker RB 1973 Food iron absorption in man. II. Isotopic exchange of iron between labeled foods and between a food and an iron salt. Am J Clin Nutr 26: 1311–1319.
Gislason J, Jones B, Lönnerdal B, Hambraeus L 1992 Iron absorption differs in piglets fed extrinsically and intrinsically59 Fe-labeled sow's milk. J Nutr 122: 1287–1292.
Hallberg L, Brune M, Rossander L 1986 Low bioavailability of carbonyl iron in man: studies on iron fortification of wheat flour. Am J Clin Nutr 43: 59–67.
Davidsson L, Mackenzie J, Kastenmayer P, Rose A, Golden BE, Aggett PJ, Hurrell RF 1996 Dietary fiber in weaning cereals: a study of the effect on stool characteristics and absorption of energy, nitrogen, and minerals in healthy infants. J Pediatr Gastroenterol Nutr 22: 167–179.
Davidsson L, Kastenmayer P, Yuen M, Lönnerdal B, Hurrell RF 1994 Influence of lactoferrin on iron absorption from human milk in infants. Pediatr Res 35: 117–124.
Bothwell TH, Charlton RW, Cook JD, Finch CA 1979 Iron absorption. In: Iron Metabolism in Man. Blackwell Scientific Publications, Oxford, pp 256–283.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Engelmann, M., Davidsson, L., Sandström, B. et al. The Influence of Meat on Nonheme Iron Absorption in Infants. Pediatr Res 43, 768–773 (1998). https://doi.org/10.1203/00006450-199806000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199806000-00009
This article is cited by
-
Iron status biomarkers in iron deficient women consuming oily fish versus red meat diet
Journal of Physiology and Biochemistry (2009)
-
Associations of iron status with dietary and other factors in 6-year-old children
European Journal of Clinical Nutrition (2007)
-
Participatory nutrition education and adoption of new feeding practices are associated with improved adequacy of complementary diets among rural Malawian children: a pilot study
European Journal of Clinical Nutrition (2005)
-
Iron status at 12 months of age — effects of body size, growth and diet in a population with high birth weight
European Journal of Clinical Nutrition (2003)
-
Postnatal iron status of Hong Kong Chinese women in a longitudinal study of maternal nutrition
European Journal of Clinical Nutrition (2001)