Abstract
The effect of the route of nutrient administration on the relative rates of leucine and phenylalanine kinetics was examined in 30 low birth weight (LBW) infants using L-[1-13C]leucine, L-[2H5]phenylalanine, and L-[2H2]tyrosine tracers. The infants received special premature formula (PF, n = 10, 117 ± 8 kcal·kg-1·d-1 and 3.2 ± 0.2 g protein·kg-1·d-1) or fortified human milk (HM,n = 10, 106 ± 6 kcal·kg-1·d-1 and 3.0 ± 0.2 g protein·kg-1·d-1), or parenteral nutrition (PN, n = 10, 80 ± 25 kcal·kg-1·d-1 and 1.8 ± 0.3 g protein·kg-1·d-1). The rate of appearance(Ra) of leucine (RaLeu), was significantly higher in group PF as compared with groups HM and PN (434 ± 51versus 377 ± 33 and 359 ± 50μmol·kg-1·h-1, p < 0.05). TheRa of phenylalanine (RaPhe) was lower in group HM as compared with group PF (94 ± 18 versus 115 ± 16,p < 0.05), RaPhe in group PN (108 ± 24μmol·kg-1·h-1) was in between group PF and HM. The relative rate of RaPhe and RaLeu expressed as RaPhe/RaLeu ratio was lower in all groups than that expected from reported whole body protein composition and from that reported in adults. The ratio of phenylalanine hydroxylation to leucine decarboxylation was 0.202 in group PF, 0.212 in group HM, and 0.161 in group PN, suggesting a higher rate of decarboxylation of leucine relative to hydroxylation of phenylalanine. We conclude that: 1) the higherRaLeu compared with the RaPhe may be the result of either a higher turnover of a body protein enriched in leucine or the consequence of higher leucine intake in infant nutrition and 2) whole body protein kinetics calculated from a single amino acid tracer do not adequately represent whole body protein metabolism.
Similar content being viewed by others
Main
The goal of nutritional management of preterm infants is the provision of nutrient intakes that will permit a prompt postnatal resumption of growth at a rate approximately that of the third trimester of intrauterine life. It is believed that this immediate growth provides the best possible conditions for subsequent normal development(1). Quality and quantity of protein energy ratio influences metabolism and the rate of growth(2, 3). Because protein synthesis is an energy-consuming process, providing sufficient protein and energy may be a key factor influencing the growth rate of LBW infants(4).
Dynamic aspects of protein metabolism have been studied using nonradioactive stable isotopic tracers of essential amino acids such as leucine and phenylalanine(5–12). These methods are based on the principle that the turnover of an essential amino acid can be used to measure protein kinetics assuming that 1 g of mixed body protein contains 590 μmol of leucine and 280 μmol of phenylalanine(13, 14). Whether these tracers give similar estimates of protein turnover has not been evaluated in infants in studies using the two tracers simultaneously. We raised the question whether in growing LBW infants the ratio of phenylalanine to leucine kinetics is identical to the theoretically proposed ratio in mixed body proteins,i.e. 280 μmol of phenylalanine/590 μmol of leucine = 0.47. In the present study, we examined the relative rates of phenylalanine and leucine kinetics simultaneously in LBW infants during enteral feeding with either PF or with HM. Because differences in the first pass uptake by the liver of leucine and phenylalanine might influence the measurement of phenylalanine and leucine kinetics in enterally fed infants, we also studied the relative rates of phenylalanine and leucine kinetics in LBW infants receiving PN.
METHODS
Infants. Thirty LBW infants, admitted to the Neonatal Intensive Care Unit of the Hospital of the Vrije Universiteit, Amsterdam, were studied. Gestational age, determined by maternal history and Dubowitz score(15), ranged between 27 and 34 wk, birth weight between 763 and 1673 g. Body weight, at the time of the tracer kinetic study, ranged between 775 and 1920 g. Clinical characteristics of the study subjects are shown in Table 1. Permission to include each infant in the study was obtained by written informed consent of the parents. The study protocol was approved by the medical ethical committee of the Hospital of the Vrije Universiteit, Amsterdam, The Netherlands.
Study protocol. Ten infants (group PF) received PF feeding(Nenatal, Nutricia, Zoetermeer, The Netherlands, 150 mL·kg-1·d-1 containing 80 kcal and 2.2 g of protein/100 mL), and 10 infants (group HM) were fed preterm human milk that was fortified with a powdered protein-mineral supplement (Nenatal Breast Milk Fortifier, Nutricia, Zoetermeer, The Netherlands). (The Breast Milk Fortifier contains 0.35 g of protein per sachet (1.5 g) with a casein to whey ratio of 40/60, and by addition of two sachets to 100 mL of human milk, the protein/energy ratio of human milk increases from 1.85 g/100 kcal to 2.5 g/100 kcal). During the tracer study the infants were receiving continuous gavage feeding by a syringe pump. The studies in groups PF and HM were performed at least 3 d after the infants were on full enteral feeding. At the time of the study, the infants in group PF and HM were clinically stable with normal renal, liver, and gastrointestinal functions, breathing spontaneously without O2 supplementation. Ten infants (group PN) were studied while receiving a PN mixture diluted with glucose 10% [mixture containing per 100 mL: 56 kcal; 8.5 g of glucose, 1.7 g of amino acids (Vaminolact), 1.7 g of fat (Intralipid)(both from Kabi Pharmacia, Stockholm, Sweden), electrolytes, vitamins, and minerals]. The infants were studied 5 d after birth, four infants were studied while on mechanical ventilatory support for respiratory insufficiency. The studies were carried out in standard incubators and did not interfere with the routine care of the infant.
Isotopes. The tracers, NaH[13C]O3,[1-13C]leucine (both 99% atom percent excess 13C, MSD, Montreal, Canada), and L-[ring-2H5]phenylalanine, L-[ring-2H4]tyrosine, and L-[ring-2H2]tyrosine (all 98% atom percent excess2 H, Cambridge Isotopes Laboratories, Andover, MA) were dissolved in saline and tested for pyrogens. Tyrosine was dissolved in saline, and the pH was adjusted to 4.7.
Breath gas analysis. Expired air was collected before and during the [1-13C]leucine infusion. Breath samples (20 mL) were collected every 10 min for 4 h. Before infusion of the label, four baseline samples were collected for determination of the natural background of13 CO2.
Infusions. The body bicarbonate pool was primed with 6.9μmol·kg-1 of NaH13CO3 as described by Van Aerdeet al.(16). At the same time primed constant infusions of [1-13C]leucine (priming dose 15μmol·kg-1, continuous 15μmol·kg-1·h-1),[2H5]phenylalanine (priming dose 6 μmol·kg-1, continuous 4 μmol·kg-1·h-1), and[2H2]tyrosine (priming dose 1.36 μmol·kg-1, continuous 1.36 μmol·kg-1·h-1) were administered for 4 h, using the same syringe pump for all infusions (Terfusion Syringe pump, STC-521, Terumo Corp., Tokyo, Japan). A priming dose of[2H4]tyrosine (0.43 μmol·kg-1) was also administered.
Four blood samples were obtained, one before and three during the last hour of the tracer infusion at 15-min intervals. Plasma was separated and stored at-20 °C until analysis.
Analysis. Enrichments ([1-13C]leucine,[2H5]phenylalanine, [2H2]tyrosine, and[2H4]tyrosine) and concentrations (unlabeled leucine, phenylalanine) were measured by gas chromatography/mass spectrometry with electron capture chemical ionization using 100-μL plasma samples. During derivatization, based on extractive alkylation, the α-amino acids were converted to methyl-formated pentafluorobenzyl derivatives. D4-leucine, D8-phenylalanine, and C9-tyrosine were used as internal standards. For the quantification of concentrations and enrichments we monitored the corresponding [M-PFB]- ([M-PFB-CH3OH]-) anions. The gas chromatography-separation analyses were performed using a Shimadzu 14A gas chromatograph (Shimadzu Corporation, Kyoto, Japan) fitted with a CP-SIL-19CB, 25 m × 0.25 mm (inside diameter) × 0.20 μm(film thickness) column (Chrompack International, Middelburg, The Netherlands) with helium as the carrier gas. Mass spectrometry detection was performed on a Kratos Concept 1H, magnetic sector mass spectrometer (Kratos Analytical, Manchester, UK) or (with similar gas chromatography conditions) an AUTOMASS 150 GC-(quadrupole)-mass spectrometry combination (Unicam Analytical Instruments B.V., Eindhoven, The Netherlands). Both mass spectrometry systems were operated under electron capture chemical ionization conditions with ammonia as the moderating gas. The precision of measurement in the ranges of enrichment, expressed as the SD in the observed mean, was better than 0.15 MPE% for all compounds measured (for n = 6 at different levels). The overall sensitivity was sufficiently high enough to study enrichments in the range of 0.2-10 MPE (%). The 13C/12C ratio of breath CO2 was measured using an isotope ratio mass spectrometer (VG OPTIMA, Fisons Instruments, Middlewich, Cheshire, UK).
The isotopic plateau was confirmed by regression analysis on the last five data points. The amino acid composition of human milk was determined after hydrolysis of protein, by HPLC, using OPA and 9-fluorenylmethoxycarbonyl as precolumn derivatization reagents. The energy content of human milk was calculated according to Fomon(17).
Calculations. The Ra of leucine(RaLeu) and of phenylalanine (RaPhe)(μmol·kg-1·h-1) were calculated by tracer dilution using the α-KIC and phenylalanine enrichment in plasma during steady state(18, 19). The rate of phenylalanine conversion to tyrosine QPhe→Tyr(μmol·kg-1·h-1) was calculated according toequation (1): where RaTyr is theRa of tyrosine (μmol·kg-1·h-1) andETyr and EPhe are the plasma enrichments of[2H5]phenylalanine and [2H4]tyrosine. The expressionRaPhe/(IPhe+RaPhe) corrects for the contribution of the infusion toRaPhe→Tyr(14). Assuming steady state conditions, calculating leucine and phenylalanine kinetics, the following equation was used: where Ra is theRa of leucine or phenylalanine, Ox is the oxidative disposal rate of leucine or the hydroxylation rate of phenylalanine to tyrosine; NOD is the NOD rate, which provides an estimate of incorporation of the amino acid into protein (synthesis); I is the rate of exogenous intake of the amino acid, and ARP is the rate of release of the amino acid from protein break down(endogenous appearance rate).
The rates of whole body protein synthesis and breakdown were calculated from leucine kinetics and phenylalanine kinetics, assuming that 1 g of body protein contains 590 μmol of leucine(20) and 280μmol of phenylalanine(14). The ratios of phenylalanine kinetics to leucine kinetics were calculated from the phenylalanine kinetics divided by the leucine kinetics derived either from plasma α-KIC enrichment (Phe/KIC) or from plasma leucine enrichment(Phe/Leu).
Statistical analysis. Data are presented as means ± SD. The one-way analysis of variance was used to compare three sets of data.t tests were used to compare the data of the three feeding groups.p values <0.05 were considered significant.
RESULTS
The clinical characteristics of the study population are displayed inTable 1. Birth weight and gestational age of the infants in group PF were lower compared with groups HM and PN. The infants in group HM were younger at the time of the tracer study than the infants in group PF. However, the body weights of the infants in group PF and HM were similar, and their daily weight gain was similar (16.0 ± 3.8 g·kg-1·d-1 group PF versus 15.2 ± 1.8 g·kg-1·d-1 group HM). The infants in all three groups were stable, and all of the infants (except four in group PN) were not on O2 supplementation and were not on antibiotics. Because the intake of the infants studies was determined independently by the physician responsible for the clinical care, the energy intake of the infants in group PF turned out to be slightly higher compared with group HM. The energy intake of the infants in group PN was lower compared with the other groups. Protein intake was similar in groups PF and HM and lower in group PN(Table 1). In all patients, a13 CO2/12CO2 plateau in expiratory air was obtained. Isotopic steady state was achieved during the study period. We found plateaus in the enrichments of plasma leucine and phenylalanine after a 4-h tracer infusion (coefficient of variation at steady state was between 0.7 and 3.7%).
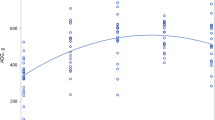
Leucine kinetics. The parameters of the leucine kinetics are presented in Table 2. As shown, the leucine intake was similar in groups PF and HM (104 μmol·kg-1·h-1) and lower in group PN (63 μmol·kg-1·h-1); however, the plasma leucine concentrations were similar in the three groups(85 ± 21 μmol·L-1, 85 ± 22μmol·L-1 and 89 ± 20 μmol·L-1). Even in the presence of a similar intake and a similar plasma level of leucine, the total Ra of leucine calculated from the α-KIC enrichment in plasma was significantly higher (p < 0.01) in group PF compared with group HM and also higher than group PN (434 ± 51μmol·kg-1·h-1 versus 377 ± 33μmol·kg-1·h-1 and 359 ± 50μmol·kg-1·h-1, p < 0.05). The calculated Ra of endogenous leucine (endogenous Ra) was significantly higher in group PF compared with group HM (331 ± 47μmol·kg-1·h-1 versus 282 ± 35μmol·kg-1·h-1, p < 0.05) and similar compared with group PN. The contribution of leucine C to CO2 was significantly higher in group PF and PN compared with group HM(p < 0.05). NOD of leucine was significantly higher in group PF compared with groups HM and PN (p < 0.05). Correspondingly the calculated rates of whole body protein breakdown and synthesis were higher in infants fed PF (p < 0.05) compared with infants fed HM and also higher compared with infants receiving nutrients parenterally.
Phenylalanine kinetics. The parameters of the phenylalanine kinetics are presented in Table 3. As shown, phenylalanine intake was significantly higher in group HM (37.8 ± 7.9μmol·kg-1·h-1) compared with groups PF and PN(30.2 ± 1.9 μmol·kg-1·h-1 and 19.2± 3.1 μmol·kg-1·h-1). The plasma phenylalanine concentrations were not different in the two enteral feeding groups (49 ± 9 μmol·L-1 group PF, 44 ± 11μmol·L-1 group HM). However, the plasma phenylalanine concentration was higher in group PN compared with groups PF and HM (61± 15 μmol·L-1, p < 0.05). The totalRa of phenylalanine (RaPhe) was significantly higher in group PF compared with groups HM and PN (145 ± 17μmol·kg-1·h-1 versus 129 ± 15μmol·kg-1·h-1 and 127 ± 22μmol·kg-1·h-1, p < 0.05). The calculated Ra of endogenous phenylalanine (endogenousRaPhe) was significantly higher in group PF compared with group HM (115 ± 16 μmol·kg-1·h-1versus 94 ± 14 μmol·kg-1·h-1,p < 0.05). The endogenous RaPhe was similar in group PF compared with group PN. The rate of hydroxylation of phenylalanine to tyrosine and the NOD rate of phenylalanine were similar in all groups.
The calculated rate of whole body protein synthesis was similar in all groups. The calculated whole body protein breakdown from phenylalanine kinetics was significantly higher in group PF (p < 0.05) compared with group HM and PN (9.8 ± 1.4 g·kg-1·d-1versus 8.1 ± 1.2 g·kg-1·d-1 and 9.2 g·kg-1·d-1).
Comparison of leucine and phenylalanine kinetics. The calculated rates of whole body protein synthesis and breakdown derived from phenylalanine kinetics were significantly lower compared with those derived from leucine kinetics (p < 0.05 in all groups). To compare the metabolism of phenylalanine and leucine in the three feeding groups, the ratios of phenylalanine to leucine intake, plasma concentrations,Ra, endogenous Ra, oxidation, and NOD were calculated(Table 4). The ratios were calculated from kinetics derived from α-KIC enrichment (Phe/KIC) and from plasma leucine enrichment (Phe/Leu). The ratio of phenylalanine intake to leucine intake was 0.293 in group PF, 0.369 in group HM, and 0.305 in group PN. The plasma phenylalanine to leucine concentration ratio was 0.564 ± 0.068 in group PF, 0.512 ± 0.076 in group HM, and 0.707 ± 0.180 in group PN. In all groups the ratios of phenylalanine to leucine (Phe/KIC) kinetics were lower than the published ratio of 0.47 (based upon published data of the composition of whole body protein in man), indicating that the leucineRa relative to phenylalanine Ra is higher in LBW infants compared with adults. The Phe/KIC ratios of the respective kinetics were similar in the three feeding groups, and higher or equal to the Phe/Leu ratio of the nutrients administered. In all instances the ratios of phenylalanine hydroxylation to leucine oxidation were significantly lower than the ratios of total Ra and also lower than the phenylalanine to leucine intake ratios (p < 0.01), suggesting a higher rate of oxidation of leucine compared with hydroxylation of phenylalanine.
DISCUSSION
In the present study we examined the effects of nutrient intake on leucine and phenylalanine kinetics measured simultaneously. To our knowledge, these are the first data that examined the effect of feeding on leucine and phenylalanine kinetics in LBW infants. Key findings of our study are:1) in LBW infants fed either enterally or parenterally the relative kinetics of phenylalanine and leucine are different compared with reported data in adults; 2) in LBW infants the ratio of quantity of phenylalanine hydroxylation and quantity of leucine decarboxylation was lower than the ratio of Ra of phenylalanine and leucine, suggesting a higher rate of decarboxylation of leucine relative to hydroxylation of phenylalanine; and 3) the Ra of leucine and phenylalanine are higher in the group fed PF compared with the group fed HM.
Considerable data exist on whole body protein kinetics in LBW infants measured with tracer [1-13C]leucine using the assumption that theRa of leucine represents protein breakdown and the Ra of leucine C into CO2 is a measure of protein oxidation(7, 9, 21). Limited studies in LBW infants have investigated phenylalanine kinetics(5, 22). Phenylalanine is catabolized almost exclusively through hydroxylation to tyrosine. No conversion from tyrosine to phenylalanine occurs. Of the minor metabolites, phenylethylamine and phenylpyruvic acid are the most important but are produced in insignificant amounts in healthy humans(23). Therefore, hydroxylation of phenylalanine to tyrosine is assumed to be a measure of protein oxidation.
In case of whole body protein kinetics calculated from phenylalanine turnover study, the assumption is made that 1 g of body protein contains 280μmol of phenylalanine, whereas in the case of leucine kinetic study, 1 g of body protein is assumed to contain 590 μmol of leucine(14, 20). Therefore, the theoretically expected ratio of phenylalanine to leucine kinetics in the whole body would be 0.47.
In the present study the ratio of total Ra of leucine and phenylalanine was 0.335 in the PF-fed group and 0.341 in the HM-fed group. This ratio was different from the expected ratio derived from the literature. Bennett et al.(24) studied in adults the ratios of total rate of release from skeletal muscle of phenylalanine and leucine (Ra). In their study the ratios were 0.43 during fasting and 0.451 during parenteral amino acid administration(24)(Table 5). Similar ratios were observed when their investigation measured whole body phenylalanine and leucine kinetics. Thompsonet al.(14) studied forearm protein turnover in adults using [1-13C]leucine and [2H5]phenylalanine. They found a Raphe/RaLeu ratio of 0.43 during fasting. In another study by Biolo et al.(25) in healthy volunteers the ratio of phenylalanine and leucine Ra was 0.46 in the basal state and 0.39 during feeding (Phe/Leu ratio in the feeding was 0.35). These ratios in fasting and fed state in healthy adults are close to the expected ratio of 0.47. In contrast, the data in newborn infants, both full-term and LBW, the ratio of Phe/Leu kinetics have been lower when compared with adults.
Denne et al.(10, 11) studied phenylalanine and leucine kinetics during fasting and feeding. They did not perform leucine and phenylalanine kinetics simultaneously in the same infants. Calculations of their data (Table 5) show aRaPhe/RaLeu ratio of 0.232 in the fasting state and 0.221 in the fed state. The lower Phe/KIC ratios in our study and those of previous studies (Tables 4 and 5) are not the consequence of a lower Phe/Leu ratio in body proteins in newborn-LBW or term infants. As shown by Widdowson et al.(26), in their studies of amino acid composition of the developing fetus, the phenylalanine/leucine ratio was 0.55 (free or bound phenylalanine and leucine). Furthermore, the lower ratios do not appear to be the consequence of nutrient administration, as similar data are derived from the previous studies in fasting newborn infants (Table 5)(10, 11). In the present study in all cases the ratios of phenylalanine kinetic parameters to leucine kinetic parameters are lower than the expected ratio of 0.47 and lower than the ratio found in the fetus(27), but higher than the ratio in the nutrients administered in group PF and group PN. In all three groups, the ratio of phenylalanine hydroxylation to leucine decarboxylation (oxidation ratio) is lower than the corresponding ratio of the Ra.
Whole body amino acid kinetics are the sum of turnover of a large number of proteins at different rates. It is assumed that the whole body represents a single large pool of mixed proteins, thus the results in the present study may be the consequence of a high turnover rate of proteins enriched with leucine relative to phenylalanine in LBW infants. Support for this argument can be presented because of a different organ to body weight ratio in infants. For example the liver to body weight ratio in infants is 0.04 as compared with 0.02 in adults. Thus a higher turnover rate in hepatic proteins compared with the whole body in newborn infants could potentially influence the whole body amino acid kinetics. In addition, the infants have larger components of other organs such as thymus and other proteins, e.g. Hb F which could also impact the measured protein kinetics. The data on the amino acid compositions of various tissue proteins are not easily available in newborn infants to make appropriate calculations. Differences in the first pass uptake by the liver of leucine and phenylalanine could also influence the measurement of phenylalanine and leucine kinetics in enterally fed infants. However, this effect can be excluded because the ratios were similar in both enterally and parenterally fed infants.
Finally, the phenylalanine hydroxylation to leucine oxidation ratio is lower compared with the RaPhe/RaLeu ratio, indicating that the leucine oxidation rate was relatively higher than the phenylalanine hydroxylation rate. The reason for that may be that the excess leucine intake may be used as a fuel source for oxidation and thus energy supply. Furthermore, because decarboxylation of C-1 of leucine also occurs when leucine enters other synthetic pathways, the higher ratio of leucine C converted to CO2 may be a reflection of leucine's participation in these synthetic processes(28). In addition, phenylalanine conversion into tyrosine could be lower in LBW infants, possibly due to the immaturity of the rate-limiting step of phenylalanine hydroxylase or enzymes of tyrosine metabolism. The phenylalanine intake was higher in group HM. However, the hydroxylation rate of phenylalanine was similar in both enteral feeding groups, possibly suggesting that a higher rate of phenylalanine intake would not induce the difference in phenylalanine hydroxylation and leucine oxidation rate.
Leucine turnover was significantly higher in the group fed PF compared with the group fed HM. An evident explanation for this difference was not found. It is known from the literature that infants born prematurely have higher rates of protein turnover compared with more mature infants(29). The infants in the PF-fed group had a lower birth weight and a lower gestational age; however, at the time of the tracer kinetic study the body weights were comparable in the two groups. Differences in the composition of the feeding regimens of the LBW infants may have influenced whole body protein kinetics.
The results of the present study indicate that the relative rates of phenylalanine to leucine kinetics in the LBW infant are different from their relative rates observed in adults. In adults the ratio of phenylalanine to leucine kinetics is close to the theoretically proposed ratio in mixed body protein both during fasting and during feeding. In contrast, the ratio of phenylalanine to leucine kinetics in LBW infants is lower. These data suggest that there is a differential contribution of various body proteins to whole body mixed protein turnover in LBW infants compared with adults. Estimates of whole body protein turnover using a single amino acid tracer do not adequately represent the dynamic aspects of whole body protein metabolism.


Abbreviations
- LBW:
-
low birth weight
- Ra :
-
rate of appearance
- NOD:
-
nonoxidative disposal
- PF:
-
preterm formula
- HM:
-
fortified human milk
- PN:
-
parenteral nutrition
- KIC:
-
ketoisocaproate
References
American Academy of Pediatrics Committee on Nutrition 1985 Nutritional needs of the low-birth-weight infants. Pediatrics 75: 976–986
Pencharz PB, Masson M, Desgrandes F, Papageorgiou A 1981; Total-body protein turnover in human premature neonates: effects of birthweight, intrauterine nutritional status and diet. Clin Sci 61: 207–215
Chessex P, Reichman B, Verellen G, Putet G, Smith JM, Heim T, Swyer PR 1984; Metabolic consequences of intrauterine growth retardation in very low birth weight infants. Pediatr Res 18: 709–713
Catzellis C, Schutz Y, Micheli JL, Welsch C, Arnaud MF, Jéquier E 1985; Whole body protein synthesis and energy expenditure in very low birth weight infants. Pediatr Res 19: 679–687
Castillo L, Yu MY, Marchini JS, Chapman TE, Sanchez M, Young Vr, Burke JF 1994; Phenylalanine and tyrosine kinetics in critically ill children with sepsis. Pediatr Res 35: 580–588
Wykes LJ, Ball RO, Menendez CE, Ginther DM, Pencharz PB 1992; Glycine, leucine and phenylalanine flux in low birth weight infants during parenteral and enteral feeding. Am J Clin Nutr 55: 971–975
Beaufrère B, Putet G, Pachiaud C, Salle B 1990; Whole body protein turnover measured with 13C-leucine and energy expenditure in preterm infants. Pediatr Res 28: 147–152
Denne SC, Karn CA, Brondyke AD, Liu YM, Liechty EA 1993; Intravenous substrate increases both protein synthesis and suppresses proteolysis in normal growing newborns. Pediatr Res 33: 302A
Beaufrère B, Fournier V, Salle B, Putet G 1992; Leucine kinetics in fed low birth weight infants: importance of splanchnic tissues. Am J Physiol 263:E214–E220
Denne SC, Karn CA, Liu YM, Leitch CA, Liechty EA 1994; Effect of enteral versus parenteral feeding on leucine kinetics and fuel utilization in premature newborns. Pediatr Res 36: 429–435
Denne SC, Karn CA, Liu YM, Liechty EA 1992; Phenylalanine kinetics in normal growing newborns during fasting and feeding. Pediatr Res 31: 287A( abstr)
Pacy PJ, Thompson GN, Halliday D 1991; Measurement of whole-body protein turnover in insulin-dependent (type 1) diabetic patients during insulin withdrawal and infusion: comparison of [13C]leucine and[2H5]phenylalanine methodologies. Clin Sci 80: 345–352
Denne SC, Rossi EM, Kalhan SC 1991; Leucine kinetics during feeding in normal newborns. Pediatr Res 30: 23–27
Thompson GN, Pacy PJ, Merritt H, Ford GC, Read MA, Cheng KN, Halliday D 1989; Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol 238:E473–E479
Dubowitz LM, Dubowitz V, Goldberg C 1970; Clinical assessment of gestational age in the newborn infant. J Pediatr 77: 1–10
Van Aerde JEE, Sauer PJJ, Pencharz PB, Canagarayar U, Beesley J, Smith JM, Swyer PR 1985; The effect of energy intake and expenditure on the recovery of 13CO2 in the neonate during 4-hour primed constant infusion of NaH13CO3 . Pediatr Res 19: 806–810
Fomon SJ 1991; Requirements and recommended dietary intakes of protein during infancy. Pediatr Res 30: 391–395
Matthews DE, Schwartz HP, Yang RD, Motil KJ, Young VR, Bier DM 1982; Relationship of plasma leucine and α-ketoisocaproate during[1-13C]leucine infusion in man; a method for measuring human intracellular leucine tracer enrichment. Metabolism 31: 1105–1112
Schwenk WF, Beaufrere V, Haymond MW 1985; Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol 249:E646–E650
Waterlow JC, Garlick PJ, Millward DJ 1978; Protein Turnover in Mammalian Tissues in the Whole Body. Elsevier North Holland, Amsterdam, pp 301–325
Van Goudoever JB, Sulkers EJ, Halliday D, Degenhart HJ, Carnielli VP, Wattimena JL, Sauer PJJ 1995; Whole body protein turnover in preterm appropriate for gestational age and small for gestational age infants: comparison of [15N]glycine and [1-13C]leucine administered simultaneously. Pediatr Res 37: 381–388
Shortland GJ, Walter JH, Fleming PJ, Halliday D 1994; Phenylalanine kinetics in sick preterm neonates with respiratory distress syndrome. Pediatr Res 36: 713–718
Scriver CR, Kaufman S, Woo SLC 1989; The hyperhenylalaninemias. In Scriver CR, Beaudet AL, Sly WS, Dalle D (eds) The Metabolic Basis of Inherited Disease. McGraw-Hill, New York, pp 497–506
Bennet WM, Connacher AA, Scrimgeour CM, Rennie MJ 1990; The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. Eur J Clin Invest 20: 41–50
Biolo G, Tessari P, Inchiostro S, Bruttomesso D, Fongher C, Sabadin L, Fratton MG, Valerio A, Tiengo A 1992; Leucine and phenylalanine kinetics during mixed meal ingestion: a multiple approach. Am J Physiol 25:E455–E463
Widdowson EM, Southgate DAT, Hey EN 1979; Body composition of the fetus and infant. In Visser HKA (ed) Nutrition and Metabolism of the Fetus and Infant. Martinus Nijhoff, The Hague, pp 169–177
Gruenwald P 1963; Chronic fetal distress and placental insufficiency. Biol Neonate 5: 215–265
Frick GP, Blinder L, Goodman HM 1988; Transamination and oxidation of leucine and valine in rat adipose tissue. J Biol Chem 263: 3245–3249
Nissim I, Yudkoff M, Pereira G, Segal S 1983; Effects of conceptual age and dietary intake on protein metabolism in premature infants. J Pediatr Gastroenterol Nutr 2: 507–516
Acknowledgements
The authors thank Wendy Guérand, Rob Kok, and Jan Meesterburrie for their expert technical assistance and Anneke Cranendonk, R.N., for her help in performing the studies.
Author information
Authors and Affiliations
Additional information
Supported by Nutricia N.V. Zoetermeer, the Netherlands. S.C.K. is supported by National Institutes of Health Grant HD 11089.
Rights and permissions
About this article
Cite this article
Van Toledo-Eppinga, L., Kalhan, S., Kulik, W. et al. Relative Kinetics of Phenylalanine and Leucine in Low Birth Weight Infants during Nutrient Administration. Pediatr Res 40, 41–46 (1996). https://doi.org/10.1203/00006450-199607000-00008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199607000-00008
This article is cited by
-
Pragmatic Approach to In-Hospital Nutrition in High-Risk Neonates
Journal of Perinatology (2005)
-
Blood Urea Nitrogen Concentration as a Marker of Amino-Acid Intolerance in Neonates with Birthweight Less than 1250 g
Journal of Perinatology (2005)