Abstract
Polyvinylidene fluoride particles and film were chemically modified by grafting with polystyrene without a preliminary treatment of the fluoropolymer surface. The modification was performed using surface-initiated atom transfer radical polymerization with Cu+–Tris (2-aminoethyl)amine as the catalytic system in a styrene/acetonitrile mixture. A small amount of Cu(II)Cl2 was added to control the polymerization process. The amount of covalently bound polystyrene was quantified using Fourier transform infrared spectroscopy, and the extent of grafting was found to be a time-dependent process at 60 °C. The formation of polystyrene brushes, which may form a uniform polystyrene layer over the surface of fluoropolymer particles, was observed via scanning electron microscopy.
Similar content being viewed by others
Introduction
Fluoropolymers, including polyvinylidene fluoride (PVDF), are important materials because of their notable chemical inertness, thermal stability and extraordinary physical properties.1 However, the chemical inertness of fluoropolymers has complicated their use in various applications where a stable coating of the polymer surface is needed. For example, an impeded stable coating of PVDF on the surface of inorganic materials such as alumina is necessary to govern certain optical and physical properties of those materials. To obtain a stable inorganic coating of PVDF, the polymer must be grafted firsthand. As several alternative procedures can be found for alumina coating, the chemical functionalization of the fluoropolymer surface has become the key technological problem.
The physical properties of PVDF can be modified by using a controlled length of PVDF chain,2 by copolymerization of PVDF with other monomers3 or by melt compounding of PVDF with inorganic nanomaterials.4 Unfortunately, these methods do not allow the modification of the surface of the polymer, which remains highly hydrophobic.
In this report, we have addressed the problem of PVDF surface grafting by using polystyrene, which is widely used to treat the surfaces of various organic and inorganic polymers and to prepare the grafted particles. The current study focuses on the grafting of PVDF microparticles to use in twisting-ball type displays.5
State of the art
Most of the known chemical methods of surface grafting have not been effective for PVDF; therefore, other hydrophilic polymers (cyanoacrylate) have been blended into the fluoropolymer to increase the hydrophilic properties of the material.6 However, this method has always reduced the quality of the material, especially if the electric properties of PVDF are considered. Therefore, we focused on the grafting of pure PVDF and analyzed the methods that could be useful for its functionalization.
In some studies, the treatment of PVDF by high-energy beams or plasma has been used.7 After the high-frequency plasma treatment, the hydrophilic properties of PVDF significantly increased, which led to varying results, including a decrease in the PVDF/H2O contact angle from 90° to 70°. The fluoropolymers were also functionalized with various oxygen-containing functional groups when they were treated with a concentrated aqueous solution of lithium hydroxide8 or an ammonia solution of lithium9 or magnesium amalgam.10 However, in all of these cases, sufficient surface activation was not achieved without significant damage to the polymer’s interior structure and without contamination of the polymer with the degradation products.
Chen et al.11 studied the direct atom transfer radical polymerization (ATRP) of poly[2-(N,N-dimethylamino)ethyl methacrylate] (PDMAEMA) and poly[poly(ethylene glycol) monomethacrylate] (PPEGMA) on the surface of PVDF films for biomedical purposes. The conventional version of the ATRP method requires the presence of an initiator and a catalyst,12 and in this process, radicals are generated through one electron oxidation of the initiator in a reversible redox process catalyzed by a transition metal-amine complex. Different amines have been used in this complex: 2,2′-bipyridine, N,N,N′,N′-tetramethylethylenediamine, 2,2′:6′,2″-terpyridine, Tris[2-(dimethylamino)ethyl]amine (Me6TREN), 1,4,8,11-tetraaza-1,4,8,11-tetramethylcyclotetradecane, and Tris[2-aminoethyl]amine (TREN). Alkyl halides RX are typically used as the initiators, and the halide group must rapidly and selectively migrate between the growing chain and the transition-metal complex. If bromine and chlorine are good leaving groups, the energy of the homolytic cleavage of the C-F bond is much higher; therefore, fluorine has generally been considered a bad leaving group in ATRP.13 This explains the lack of data about C-F bond participation in polymerization reactions of this type in the literature, except for the study cited above,11 where PDMAEMA and PPEGMA were grafted to PVDF by using the ATRP mechanism with polymer initiation at the secondary fluorinated site.
Following this lead, we developed a chemical method to modify the PVDF surface with polystyrene ‘brushes,’ which grow from the fluoropolymer surface where mono-functional initiator sites are activated. In the absence of a thermal self-initiation and a chain transfer phenomena, this ‘grafting from’ process was easy to control. It also yielded a uniform and high-quality polystyrene layer.
Moreover, a systematic study of the surface modification process revealed another option that had a minor effect on the present technology but could be a valuable lead to develop a new ‘grafting chemistry’ of fluorinated polymers, in which the molecules contain hydrogen atoms alternating with fluorine atoms. Hypothetically, these fluoropolymers could behave as CH acids because the dissociation in the presence of bases may give rise to ionic polymerization reactions. In principle, the ATRP initiated radical polymerization and the hypothetical base-promoted ionic polymerization reactions may even occur in parallel if the reaction conditions are similar.
Experimental Procedure
Materials
PVDF particles with a diameter of 50 μm were tailored by Solvay Solexis (Milano, Italy), and their mean molecular masses were 31 500 or 315 000 for the two lots used in this study. In some experiments, PVDF film (thickness 150 μm) was used. Before the experiments, the surface of the specimen was cleaned by first washing with methanol and then with CCl4.
The chemicals in our experiments were purchased from Sigma Aldrich (St Louis, MO, USA) as American Chemical Society (ACS) grade reagents and were used without additional purification, except styrene and acetonitrile. Styrene was distilled before the experiments (18 mm Hg bp 44 °C), and the stabilizer (hydroquinone) was removed by inhibitor remover 311322 (CAS 9003-70-7, Aldrich). No hydroquinone was detected in the purified styrene by fourier transform infrared (FT-IR) spectral analysis. Acetonitrile was dried over molecular sieves. CuCl2 was purified by precipitation from glacial acetic acid, followed by washing with 2-propanol. CuCl was at the desired purity level (99.995%). All salts were kept in vacuum desiccators.
Attenuated total reflection-FT-IR spectra
Spectra of both the surface-functionalized material and the blank material were obtained using attenuated total reflection-FT-IR spectroscopy with a Nicolet 6700 FT-IR spectrometer, which was calibrated against air before each series of experiments. Each spectrum was collected by accumulating 256 scans at a resolution of 4 cm−1, and three sample readings from different surface locations were recorded to eliminate the effects of micro-heterogeneity.
Scanning electron microscopy (SEM)
A small amount of powder was dispersed in the EpoThin epoxy resin (Buehler Inc., Lake Bluff, IL, USA) and cured in small molds at room temperature. Slices with a thickness of 5 μm were cut with an ultramicrotome LKB ULTROTOME V (LKB Produkter, Stockholm, Sweden), attached to the aluminum stubs and coated with Au/Pd by ion sputtering to enhance the electrical conductivity. The samples were examined with a scanning electron microscope (ZEISS EVO MA15, Carl Zeiss Inc., Oberkochen, Germany) at 10 kV. For the given samples, each SEM image presented in this paper is a representative of numerous images made at different locations of the sample.
Synthesis of the catalyst
Synthesis of the ATRP catalyst was accomplished in an oxygen-free atmosphere using dry-box equipment. In brief, acetonitrile was bubbled for 30 min with dry argon gas, and the components were added to obtain the following solution: [TREN]=167 mM, [CuCl]=45 mM and [CuCl2]=4,5 mM or 9.0 mM. This mixture was stirred for ∼24 h at 30 °C in an argon atmosphere. The catalyst was stored at 4 °C in an airtight dry-box freezer.
PVDF grafting with polystyrene
The ATRP reactions were made in the following mixtures: 2.5 ml styrene (4.86 M), 1.5 ml acetonitrile, 0.5 ml of catalyst in acetonitrile, 50 mg of PVDF samples (spherical particles or film, molecular weight: 315 000 or 31 000). The samples were stirred at 60 °C in an argon atmosphere. The reactions were stopped by rapid cooling of the reaction mixture to room temperature and simultaneous exposure to air.
In some test experiments, copper salts and TREN were omitted from the reaction mixture, and 0.5 ml of acetonitrile was added in these cases. In other test experiments, copper salts were omitted, and 0.5 ml of pure TREN was added, yielding a concentration of 0.75 M in the reaction mixture.
As we were interested in the covalently attached polystyrene, the particles were carefully washed three times with CCl4 before spectroscopic measurements. This treatment was sufficient to remove traces of polystyrene and styrene from the surface of the material because increasing the number of washes had no effect on the results. After this treatment, the FT-IR spectra were monitored, and the absorbance at 699 cm−1 was considered an indication of the presence of grafted polystyrene. This absorbance corresponds to the C-H out of plane bending in monoalkylated aromatics and comprises the strongest absorption band of styrene.14
The ratio between the height of this absorption band and that of the absorption band for CF vibrations of PVDF at 1178 cm−1 (Simoes et al.)15 (CH/CF) was used to estimate the amount of grafted polystyrene in arbitrary units, provisionally marked as the ‘grafting yield’.16
Results and discussion
ATRP of styrene on PVDF surface
Using the CuCl/CuCl2 complex with TREN as the catalyst, we were able to initiate polystyrene grafting on the surface of the spherical PVDF particles, which were produced by emulsion polymerization in Solvay Solexis. A significant amount of covalently bound polystyrene was detected after 1 h, and the results were similar for the PVDF samples of molecular weight: 31 000 and 315 000. Under the same conditions, we were unable to observe the grafting on PVDF film, and the covalently bound polystyrene appeared only after a significant extension of the reaction time. A plausible explanation for this result is that the spherical particles have a larger net surface area than pressed film of the same mass. This difference affected the rate of initiation of ATRP on the PVDF surface. However, because we focused on the grafting of spherical particles, this result satisfied our objectives, and all further results described were obtained with spherical PVDF particles.
As previously mentioned, the covalently attachment of polystyrene was ascertained using the attenuated total reflection-FT-IR technique,14 where the separation of non-covalently bound polystyrene from the grafted polymer has a special importance. Therefore, a separate study of the sample treatment conditions was made. First, the samples were washed extensively with CCl4, which is an efficient solvent for polystyrene. Then, the washed grafted polymer samples were left in this solvent overnight before the spectra were measured. Finally, the washed samples were treated with ultrasound. After these treatments, the PVDF particles still retained their characteristic polystyrene peaks in the FT-IR spectra, which confirmed the covalent attachment of polystyrene to the PVDF surface.
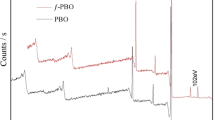
The FT-IR spectra were also used to investigate the time course of the grafting reaction. For example, absorption at 699 cm−1 was absent in the initial PVDF particles (spectrum A, Figure 1), but this band was observed after the grafting reaction progressed for 15, 30 and 60 min (spectra B, C and D in Figure 1).
Changes in the FT-IP spectra demonstrate PVDF grafting with polystyrene. (a) PVDF particles before grafting; (b–d) PVDF particles after grafting for 15 min, 30 min and 60 min. The increase in the peak at 699 cm−1, which was the strongest polystyrene absorption band, demonstrated the development of polymer brushes on the surface of PVDF particles. Conditions of the reaction: 50 mg of PVDF particles, molecular weight: 315 000; [styrene]=4.86. M; [AcN]=19.5 M, [TREN]=19 mM, [CuCl]=5 mM; [CuCl2]=0.5 mM, and temperature 60 °C. A full color version of this figure is available at Polymer Journal online.
Morphology of grafted PVDF particles surface
The covalent attachment of polystyrene to PVDF significantly changed the surface morphology of the PVDF particles as observed by specimen probing with scanning electron microcopy (Figure 2). Figure 2a shows that the starting material possessed a nearly smooth surface with no significant defects. The formation of polystyrene dendrites was visible on the surface after the treatment with styrene in the presence of an ATRP catalyst (Figure 2b). The polystyrene ‘brushes’ uniformly covered the surface of the particle. A magnified image of these dendrites is given in Figure 3.
The density of these ‘brushes’ and their dimensions increased with reaction time. The rate of growth also depended on the composition of the catalyst. Therefore, an appropriate reaction time and other conditions can be chosen to obtain a grafting polymer layer of the required dimensions. These choices depend on the technological application of the grafted polymer.
Time course of the surface modification
Using the proposed spectroscopic method to determine the covalently bound polystyrene, the time course of the grafting process was monitored at different reaction conditions. In Figure 4, the influence of the different ratios of Cu(I) and Cu(II) concentrations on the rate of the process is compared (curves A and B). It can be seen that increasing the content of CuCl2 decreased the initial rate of the process. This correlation agrees with the general ATRP mechanism, in which the equilibrium between different oxidation states of the copper catalyst should determine the concentration of the reactive species (the number of initiator sites that are formed); therefore, the equilibrium should also govern the initial rate of the catalyzed reaction. This situation is illustrated in reaction Scheme 1, in which formation of the initiator sites through the homolytic cleavage of C-F bonds was assumed. This initiation process was catalyzed by Cu(I) salt, and the concentration was regulated through its equilibrium with Cu(II) salt.
Time course of polystyrene covalent attachment to PVDF particles at 60 °C, as assessed by the ratio of polystyrene and PVDF characteristic absorbances at 699 and 1178 cm−1, respectively. Different amounts of Cu(I)Cl and Cu(II)Cl2 were used in the experiments. Curve A: [CuCl]=5 mM; [CuCl2]=0.5 mM, [TREN]=19 mM. Curve B: [CuCl]=5 mM; [CuCl2]=1 mM, [TREN]=19 mM. Curve C: no copper salts, [TREN]=760 mM.
Furthermore, Figure 4 shows that the polymerization reaction leveled off after a certain time interval, and the time course of the process was modeled by an exponential function. Interestingly, both ATRP processes had similar half-lives, although the time spans and the initial rates were different. Without an in-depth discussion of the kinetics of these processes, it can be noted that these regularities allow one to control the mass and the dimensions of the grafted polystyrene layer, which is an important feature of the discussed ATRP technology.
Surface-initiated anionic polymerization
However, in addition to the conventional ATRP reaction described above, the covalent attachment of polystyrene to the PVDF surface was also observed in experiments in which the copper catalyst was omitted from the reaction mixture but a large amount of the catalytic ligand TREN was added (760 mM). Evidently, this reaction was not initiated by the ATRP mechanism. Explaining the role of TREN in this reaction, a hypothetical mechanism for this process is suggested in Scheme 2. In formulating this mechanism, we took into account that TREN is a strong base that can initiate CH acid dissociation and carbanion formation from the PVDF chain; thus, TREN can initiate the anionic polymerization of styrene. The formation of carbanions through CH bond heterolytic dissociation should be strongly supported by neighboring fluorine atoms in the PVDF structure because it is typical for fluorine atoms to influence the CH bond strength in structural fragments of the form F-C-C-H.17
After initiation, the polymerization reaction may lead to the formation of polystyrene brushes on the PVDF surface, and these brushes are detected in the FT-IR spectrum. However, unlike the ATRP mechanism, this putative carbanion formation mechanism has not yet been studied in detail, but it may provide new perspectives on the grafting of fluorinated polymeric materials.
Conclusions
The application of typical ATRP conditions allowed the chemical functionalization of a PVDF surface and the grafting of spherical particles of this material with polystyrene. The copper catalyst, consisting of CuCl and CuCl2 in complex with TREN was used to initiate the process. The rate of the grafting process and the properties of the polystyrene layer were governed by the conditions of the process, and they could be controlled by varying the ratio of copper salts, which are used to catalyze the formation of the polymerization sites and by selecting an appropriate reaction time. These results agreed with the general mechanism of ATRP reactions and provided a good possibility for optimizing the surface functionalization technology, which is necessary to improve the optical properties of PVDF particles.
The same study also revealed another possibility for initiating polystyrene grafting on a PVDF surface. This reaction occurred without a copper catalyst, but an excess of TREN was needed. We propose that this process occurs via carbanion formation through the dissociation of C-H bonds, which are activated by neighboring fluorine atoms.

Formation of radical initiator sites through homolytic cleavage of C-F bonds, followed by styrene polymerization starting from these radicals formed on the PVDF surface. The initiation step was catalyzed by Cu(I)Cl/ligand complex following the ATRP mechanism.

Formation of anionic initiator sites through CH bond heterolytic dissociation, assisted by the basic ligand and supported by neighboring fluorine atoms in PVDF structure, and followed by styrene polymerization starting from these carbanion sites formed on the PVDF surface.
References
Foster, F. S., Harasiewicz, K. A. & Sherar, M. D. A. History of medical and biological imaging with polyvinylidene fluoride (pvdf) transducers. Ultrasonics, Ferroelectr Frequency Control, IEEE Trans 47, 1363 (2000).
Durand, N., Ameduri, B., Takashima, K., Ishida, K., Horie, S. & Ueda, Y. Vinylidene fluoride telomers for piezoelectric devices. Pol. J. 43, 171–179 (2011).
Vidhate, S., Chung, J., Vaidyanathan, V. & D’Souza, N. A. Resistive-conductive transitions in the time-dependent piezoresponse of PVDF-MWCNT nanocomposites. Pol. J. 42, 567–574 (2010).
Yamada, E., Nishioka, A., Suzuki, H., Murasawa, G., Miyata, K., Koda, T. & Ikeda, S. Effect of blended montomollironite on crystallization of poly(vinylidene fluoride). Pol. J. 41, 383–388 (2009).
Sheridon, N. K. Twisting ball panel display. US Patent US4126854 (1978).
Megaridi, C., Bayer, I. S. & Tiwari, M. K. Polymer composite formulations from poly(vinylidene fluoride) (PVDF) and cyanoacrylates (CA) and methods for use in large-area. WIPO Patent WO/2009/158046 (2009).
Buonomenna, M. G., Lopez, L. C., Favia, P., d'Agostino, R., Gordano, A. & Drioli, E. New PVDF membranes: the effect of plasma surface modification on retention in nanofiltration of aqueous solution containing organic compounds. Water Research 41, 19 (2007).
Crowe, R. & Badyal, J. P. S. Surface modification of poly(vinylidene difluoride) (PVDF) by LiOH. J. Chem. Soc., Chem. Commun. 958–959 (1991).
Chakrabarti, N. & Jacobus, J. The chemical reduction of poly(tetrafluoroethylene). Macromolecules 21, 3011 (1988).
Kavan, L., Janda, P. & Weber, J. Surface modification of poly(tetrafluoroethylene) by magnesium amalgam. J. Mat. Sci. 36, 879 (2001).
Chen, Y., Liu, D., Deng, Q., He, X. & Wang, X. Atom transfer radical polymerization directly from poly(vinylidene fluoride): surface and antifouling properties. J. Polym. Sci.: Polym. Chem. 44, 3434–3443 (2006).
Matyjaszewski, K. http://www.cmu.edu/maty/chem/index.html (accessed 3 June 2011).
Matyjaszewski, K. & Jianhui, X. Atom transfer radical polymerization. Chem. Rev. 101, 2924 (2001).
Masson, J.-F., Pelletier, L. & Collins, P. Rapid FTIR method for quantification of styrene-butadiene type copolymers in bitumen. J. Appl. Polym. Sci. 79, 1034–1041 (2001).
Simoes, R. D., Rodriguez-Perez, M. A., De Saja, J. A. & Constantino, C. J. L. Tailoring the structural properties of PVDF and P(VDF-TrFE) by using natural polymers as additives. Polym. Eng. Sci. 49, 2150–2157 (2009).
Albores-Velasco, M. & Lopez, E. G. R. Quantification of polystyrene acylation by IR spectroscopy an experiment on polymer IR analysis. J. Chem. Edu. 74, 551 (1997).
Burgess, D. R., Zachariah, M. R., Tsang, W. & Westmoreland, P. R. Thermochemical and chemical kinetic data for fluorinated hydrocarbons. Prog. Energy Combust. Sci. 21, 453–529 (1996).
Acknowledgements
This work was supported by the Enterprise Estonia grant EU28736. Special thanks to Dr Bernard Goffaux from Solvay Solexis for the raw materials and samples. Thanks to Dr Urve Kallavus from Tallinn Material Research Center for her assistance with the SEM microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liiv, J., Zekker, I., Panov, D. et al. Chemical functionalization of a polyvinylidene fluoride surface. Polym J 45, 313–317 (2013). https://doi.org/10.1038/pj.2012.148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2012.148