Abstract
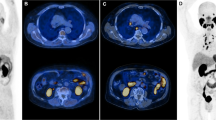
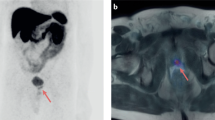
In this pilot study, the predictive value of Octreotide scintigraphy (Octreoscan) and/or Chromogranin-A (CgA) was investigated in patients with hormone-refractory prostate cancer treated with Octreotide acetate. In total, 20 patients with progressive disease and bone metastases entered the trial. At baseline Octreoscan, CgA, PSA, alkaline phosphates (ALP) and two self-administered questionnaires (EORTC QLQ C-30 (v3) and brief pain index) were performed and a diary of the pharmaceutical was started. The treatment consisted of Octreotide (Sandostatin LAR) acetate 30 mg intramuscular injection every month. The blood samples and questionnaires were repeated every month until 3 months. Clinical responder was defined as a patient with increased global health score more than 10 units and stable or decreased pain score without an increase in analgesic. In all, 17 patients were treated per protocol, and four were assessed as clinical responders. Six patients developed a reduction in ALP (median −26%, range −5 to −78%). All patients increased in PSA. At baseline, three patients had a negative Octreoscan and the patients with positive lesions, demonstrated uptake of low intensity. At baseline the CgA was elevated above the normal range in 15 of the patients, and during treatment five patients decreased their CgA to the normal range. Neither baseline Octreoscan nor CgA could identify the clinical reponders. A minority of patients improves their health-related quality of life. The decrease and normalization of CgA levels in five patients during therapy indicates therapeutic activity but Octreoscan and CgA could not identify clinical responders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Levi F, Lucchini F, Negri E, Boyle P, Vecchia CL . Leveling of prostate cancer mortality in Western Europe. Prostate 2004; 60: 46–52.
Statistics-Health and Diseases. Causes of death 2001. Stockholm, Sweden: the National Board of Health and Welfare. Centre for Epidemiology 2003.
Petrylak DP, Tangen CM, Hussain MH, Lara Jr. PN, Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
Hansson J, Abrahamsson PA . Neuroendocrine differentiation in prostatic carcinoma. Scand J Urol Nephrol 2003; (Suppl): 28–36.
Berruti A, Dogliotti L, Mosca A, Tarabuzzi R, Torta M, Mari M et al. Effects of the somatostatin analog lanreotide on the circulating levels of chromogranin-A, prostate-specific antigen, and insulin-like growth factor-1 in advanced prostate cancer patients. Prostate 2001; 47: 205–211.
Dupont A, Boucher H, Cusan L, Lacourciere Y, Emond J, Labrie F . Octreotide and bromocriptine in patients with stage D2 prostate cancer who relapsed during treatment with flutamide and castration. Eur J Cancer 1990; 26: 770–771.
Figg WD, Thibault A, Cooper MR, Reid R, Headlee D, Dawson N et al. A phase I study of the somatostatin analogue somatuline in patients with metastatic hormone-refractory prostate cancer. Cancer 1995; 75: 2159–2164.
Kalkner KM, Nilsson S, Westlin JE . [111In-DTPA-D-Phe1]-octreotide scintigraphy in patients with hormone-refractory prostatic adenocarcinoma can predict therapy outcome with octreotide treatment: a pilot study. Anticancer Res 1998; 18: 513–516.
Logothetis CJ, Hossan EA, Smith TL . SMS 201–995 in the treatment of refractory prostatic carcinoma. Anticancer Res 1994; 14: 2731–2734.
Maulard C, Richaud P, Droz JP, Jessueld D, Dufour-Esquerre F, Housset M . Phase I–II study of the somatostatin analogue lanreotide in hormone-refractory prostate cancer. Cancer Chemother Pharmacol 1995; 36: 259–262.
Verhelst J, De Longueville M, Ongena P, Denis L, Mahler C . Octreotide in advanced prostatic cancer relapsing under hormonal treatment. Acta Urol Belg 1994; 62: 83–88.
Janson ET, Westlin JE, Eriksson B, Ahlstrom H, Nilsson S, Oberg K . 111In-DTPA-D-Phe1]octreotide scintigraphy in patients with carcinoid tumours: the predictive value for somatostatin analogue treatment. Eur J Endocrinol 1994; 131: 577–581.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J . Interpreting the significance of changes in helath-related quality-of-life scores. J Clin Oncol 1998; 16: 139–144.
Cleeland CS . Measurement of pain by subjective report. In: Chapman CR, Loeser JD (eds). Advances in Pain Research and Therapy. Issues in Pain Measurement, vol. 12. Raven Press: New York, 1989, pp. 391–403.
Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988; 61: 195–202.
Krenning EP, de Jong M, Kooij PP, Breeman WA, Bakker WH, de Herder WW et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol 1999; 10: S23–S29.
Stridsberg M, Hellman U, Wilander E, Lundqvist G, Hellsing K, Oberg K . Fragments of chromogranin A are present in the urine of patients with carcinoid tumours: development of a specific radioimmunoassay for chromogranin A and its fragments. J Endocrinol 1993; 139: 329–337.
Patel YC . Somatostatin and its receptor family. Front Neuroendocrinol 1999; 20: 157–198.
Brevini TA, Bianchi R, Motta M . Direct inhibitory effect of somatostatin on the growth of the human prostatic cancer cell line LNCaP: possible mechanism of action. J Clin Endocrinol Metab 1993; 77: 626–631.
Pinski J, Halmos G, Schally AV . Somatostatin analog RC-160 and bombesin/gastrin-releasing peptide antagonist RC-3095 inhibit the growth of androgen-independent DU-145 human prostate cancer line in nude mice. Cancer Lett 1993; 71: 189–196.
Koppan M, Nagy A, Schally AV, Arencibia JM, Plonowski A, Halmos G . Targeted cytotoxic analogue of somatostatin AN-238 inhibits growth of androgen-independent dunning R-3327-AT-1 prostate cancer in rats at nontoxic doses. Cancer Res 1998; 58: 4132–4137.
Reubi JC, Waser B, Schaer JC, Markwalder R . Somatostatin receptors in human prostate and prostate cancer. J Clin Endocrinol Metab 1995; 80: 2806–2814.
Nilsson S, Reubi JC, Kalkner KM, Laissue JA, Horisberger U, Olerud C et al. Metastatic hormone-refractory prostatic adenocarcinoma expresses somatostatin receptors and is visualized in vivo by [111In]-labeled DTPA-D-[Phe1]-octreotide scintigraphy. Cancer Res 1995; 55: 5805s–5810s.
Hansson J, Bjartell A, Gadaleanu V, Dizeyi N, Abrahamsson PA . Expression of somatostatin receptor subtypes 2 and 4 in human benign prostatic hyperplasia and prostatic cancer. Prostate 2002; 53: 50–59.
Reinartz P, Schaffeldt J, Sabri O, Zimny M, Nowak B, Ostwald E et al. Benign versus malignant osseous lesions in the lumbar vertebrae: differentiation by means of bone SPET. Eur J Nucl Med 2000; 27: 721–726.
Angelsen A, Syversen U, Stridsberg M, Haugen OA, Mjolnerod OK, Waldum HL . Use of neuroendocrine serum markers in the follow-up of patients with cancer of the prostate. Neuroendocrine differentiation in carcinomas of the prostate: do neuroendocrine serum markers reflect immunohistochemical findings? Prostate 1997; 31: 110–117.
Berruti A, Mosca A, Tucci M, Terrone C, Torta M, Tarabuzzi R et al. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer 2005; 12: 109–117.
Acknowledgements
Grants from Novartis AB, Sweden supported this report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalkner, K., Acosta, S., Thorsson, O. et al. Octreotide scintigraphy and Chromogranin A do not predict clinical response in patients with octreotide acetate-treated hormone-refractory prostate cancer. Prostate Cancer Prostatic Dis 9, 92–98 (2006). https://doi.org/10.1038/sj.pcan.4500843
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500843
Keywords
This article is cited by
-
SSTR-based theranostics in neuroendocrine prostate cancer (NEPC)
Clinical and Translational Imaging (2022)