Abstract
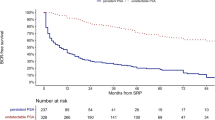
Detectable prostate-specific antigen levels (PSA) following radical prostatectomy (RP) are believed to represent treatment failure. In this retrospective review, we characterize long-term PSA outcomes following RP (n=204) in a nonreferral hospital performed between 1984 and 1994. With an average follow-up of 10 y, 90 (44%) patients developed a PSA recurrence: 15 (17%) died of prostate cancer despite hormonal intervention, 39 (43%) responded to hormonal therapy with stable remission and 36 (40%) were observed without intervention. Following RP many patients may have a detectable PSA that does not require treatment. PSA doubling time (<12 months) was the best predictor of disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Catalona WJ et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991; 324: 1156–1161.
Potosky AL, Miller BA, Albertsen PC, Kramer BS . The role of increasing detection in the rising incidence of prostate cancer. JAMA 1995; 273: 548–552.
Eastham JA, Scardino PT . Radical Prostatectomy. In: Walsh PC, Retik AB, Vaughn Jr ED, Wein AJ (eds). Campbell's Urology, 8th ed, WB Saunders, Philadelphia, PA, 2002, pp 3094–3101.
Partin AW et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA 1997; 277: 1445–1451.
Kattan MW et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998; 90: 766–771.
Trapasso JG, deKernion JB, Smith RB, Dorey F . The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol 1994; 152: 1821–1825.
Partin AW et al. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology 1994; 43: 649–659.
Patel A, Dorey F, Franklin J, deKernion JB . Recurrence patterns after radical retropubic prostatectomy: clinical usefulness of prostate specific antigen doubling times and log slope prostate specific antigen. J Urol 1997; 158: 1441–1445.
Cheng L et al. Correlation of margin status and extraprostatic extension with progression of prostate carcinoma. Cancer 1999; 86: 1775–1782.
Jhaveri FM et al. Declining rates of extracapsular extension after radical prostatectomy: evidence for continued stage migration. J Clin Oncol 1999; 17: 3167–3172.
Stamey TA et al. Histological and clinical findings in 896 consecutive prostates treated only with radical retropubic prostatectomy: epidemiologic significance of annual changes. J Urol 1998; 160: 2412–2417.
Amling CL et al. Influence of prostate-specific antigen testing on the spectrum of patients with prostate cancer undergoing radical prostatectomy at a large referral practice. Mayo Clin Proc 1998; 73: 401–406.
Catalona WJ, Smith DS, Ratliff TL, Basler JW . Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA 1993; 270: 948–954.
Han M et al. Era specific biochemical recurrence free survival following radical prostatectomy for clinically localized prostate cancer. J Urol 2001; 166: 416–419.
Gerber GS et al. Results of radical prostatectomy in men with clinically localized prostate cancer. JAMA 1996; 276: 615–619.
Lu-Yao GL, Yao SL . Population-based study of long-term survival in patients with clinically localised prostate cancer. Lancet 1997; 349: 906–910.
Messing E et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node positive prostate cancer. N Engl J Med 1999; 341: 1781–1788.
The Medical Research Council Prostate Cancer Working Party Investigators Group . Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council trial. Br J Urol 1997; 79: 235–246.
Myers RP et al. Hormonal treatment at time of radical retropubic prostatectomy for stage D1 prostate cancer: results of long-term followup. J Urol 1992; 147: 910–915.
Koch MO et al. Characterization and predictors of prostate specific antigen progression rates after radical retropubic prostatectomy. J Urol 2000; 164: 749–753.
Pound CR et al. Natural History of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281: 1591–1597.
Pruthi RS, Johnstone I, Tu IP, Stamey TA . Prostate-specific antigen doubling times in patients who have failed radical prostatectomy: correlation with histologic characteristics of the primary cancer. Urology 1997; 49: 737–742.
Soergel TM et al. Accuracy of predicting long-term prostate specific antigen outcome based on early prostate specific antigen recurrence results after radical prostatectomy. J Urol 2001; 166: 2198–2201.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahlering, T., Skarecky, D. Long-term outcome of detectable PSA levels after radical prostatectomy. Prostate Cancer Prostatic Dis 8, 163–166 (2005). https://doi.org/10.1038/sj.pcan.4500788
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500788
Keywords
This article is cited by
-
Recurrence of prostate cancer after radical prostatectomy in a 57-year-old man
Current Urology Reports (2008)