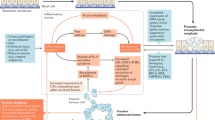
Abstract
Differences in the incidence of prostate cancer (CaP) amongst different migrant populations point to causative agents of dietary and/or environmental origin. Prostate tissues were obtained following transurethral resection of the prostate (TURP) or radical retropubic prostatectomy. After surgery, TURP-derived or tumour-adjacent tissue fragments were minced in warm PFMR-4A medium (37°C) and suspensions pipetted into collagen-coated petri dishes. Non-adherent material was removed by washing with fresh medium after 12 h. Adhered cells subsequently reacted positively with monoclonal antibodies to prostate specific antigen (PSA). PSA was also detected in the medium. The genotoxicities of the chemical carcinogens 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP), its N-hydroxy metabolite (N-OH-PhIP) and benzo[a]pyrene (B[a]P) in adherent cell populations from different donors (n=8) were examined. Cells were treated in suspension for 30 min at 37°C in the presence of the DNA repair inhibitors hydroxyurea (HU) and cytosine arabinoside (ara-C). DNA single-strand breaks were detected in cells by the alkaline single cell-gel electrophoresis (‘Comet’) assay and quantified by measuring comet tail length (CTL) in μm. All three carcinogens induced dose-related increases in CTLs (P<0.0001) in cells from four donors 24 h post-seeding. However, in cells from a further two donors the genotoxic effects of PhIP, N-OH-PhIP and B[a]P were much less apparent after 48 h than after 24 h in culture. After 96 h in culture, cells from these donors appeared to be resistant to the comet-forming activity of the compounds. However, B[a]P-DNA adducts were still measurable by 32P-postlabelling for up to 14 days following a 24-h exposure to 50 μM B[a]P in adhered cells from another two donors. This study shows that primary cultures of cells derived from the prostate can activate members of two classes of chemical carcinogens. Further development may provide a robust model system in which to investigate the aetiology of CaP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peehl DM . Culture of human prostatic epithelial cells In: Freshney RI (ed) Culture of Epithelial Cells Wiley-Liss: New York 1992 159–180
McNeal JE . The prostate gland: morphology and pathobiology Monograph Urol 1988 9: 36–63
Office for National Statistics Series DH2. No 23. Mortality Statistics by Cause: England and Wales 1996 Her Majesty's Stationery Office: London 1996
American Cancer Society. Cancer Facts and Figures—1991 Atlanta: ACS 1991 p 5
Cancer Statistics in Japan—1997. In: Kakizoe T (ed) Foundation of Promotion on Cancer Research Tokyo 1997 32–47
Muir CS, Nectoux J, Staszewski J . The epidemiology of prostatic cancer Acta Oncol 1991 30: 133–140
Locke FB, King H . Cancer mortality risk among Japanese in the United States J Natl Cancer Inst 1980 65: 1149–1156
Yu H et al. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States Int J Epidemiol 1991 20: 76–81
Shimizu H et al. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County Br J Cancer 1991 63: 963–966
Platz EA et al. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals J Natl Cancer Inst 2000 92: 2009–2017
Parkin DM, Pisani P, Ferlay J . Estimates of the worldwide incidence of 25 major cancers in 1990 Int J Cancer 1999 80: 827–841
Gayther SA et al. The frequency of germ-line mutations in the breast cancer predisposition genes BRCA1 and BRCA2 in familial prostate cancer Cancer Res 2000 60: 4513–4518
Dunsmuir WD, Hrouda D, Kirby RS . Malignant changes in the prostate with ageing Br J Urol 1998 82: (Suppl 1) 47–58
WHO. The World Health Report World Health Organization: Geneva, Switzerland 1997
Knize MG, Salmon CP, Pais P, Felton JS . Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens Adv Exp Med Biol 1999 459: 179–193
Shirai T et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods Cancer Res 1997 57: 195–198
Snyderwine EG . Some perspectives on the nutritional aspects of breast cancer research. Food derived heterocyclic amines as etiologic agents in human mammary cancer Cancer 1994 74: 1070–1077
Felton JS, Knize MJ . Occurrence, identification, and bacterial mutagenicity of heterocyclic amines in cooked food Mutat Res 1991 259: 205–217
Turesky RJ . DNA adducts of heterocyclic aromatic amines, arylazides and 4-nitroquinoline 1-oxide In: Hemminki K, Dipple A, Shuker DEG, Kadlubar FF, Segerbäck D, Bartsch H (eds) DNA Adducts—Identification and Biological Significance IARC Scientific Publications: IARC, Lyon 1994 125: S217–S228
Pfau W et al. Identification of the major hepatic DNA adduct formed by the food mutagen 2-amino-9H-pyrido[2,3-b]indole (AαC) Chem Res Toxicol 1997 10: 1192–1197
Moore RA, Melchionna RH . Production of tumors of the prostate of the white rat with 1,2-benzpyrene Am J Cancer 1937 30: 731–741
Sims P et al. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide Nature 1974 252: 326–328
Williams JA et al. Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue Carcinogenesis 2000 21: 1683–1689
Wang CY et al. N-Acetyltransferase expression and DNA binding of N-hydroxyheterocyclic amines in human prostate epithelium Carcinogenesis 1999 20: 1591–1595
Martin FL et al. DNA damage in breast epithelial cells: detection by the single-cell gel (comet) assay and induction by human mammary lipid extracts Carcinogenesis 1997 18: 2299–2305
Martin FL et al. The DNA repair inhibitors hydroxyurea and cytosine arabinoside enhance the sensitivity of the alkaline single-cell gel electrophoresis (‘comet’) assay in metabolically-competent MCL-5 cells Mutat Res 1999 445: 21–43
Reddy MV, Randerath KR . Nuclease P1 mediated enhancement of sensitivity of 32P-post-labelling test for structurally diverse DNA adducts Carcinogenesis 1986 7: 1543–1551
Doll R . Nature and nurture: possibilities for cancer control Carcinogenesis 1996 17: 177–184
Martin FL . Genotoxins and the initiation of sporadic breast cancer Mutagenesis 2001 16: 101–107
Phillips DH . Polycyclic aromatic hydrocarbons in the diet Mutat Res 1999 443: 139–147
Pfau W et al. Heterocyclic aromatic amines induce DNA strand breaks and cell transformation Carcinogenesis 1999 20: 545–551
Nadon L et al. Cancer risk due to occupational exposure to polycyclic aromatic hydrocarbons Am J Ind Med 1995 28: 303–324
Tolbert PE . Oils and cancer Cancer Causes Control 1997 8: 386–405
Krstev S et al. Occupational risk factors and prostate cancer in US blacks and whites Am J Ind Med 1998 34: 421–430
Shirai T et al. Experimental prostate carcinogenesis-rodent models Mutat Res 2000 462: 219–226
Her C et al. Human hydroxysteroid sulfotransferase SULT2B1: two enzymes encoded by a single chromosome 19 gene Genomics 1998 53: 284–295
Hayward SW, Del Buono R, Deshpande N, Hall PA . A functional model of adult human prostate epithelium. The role of androgens and stroma in architectural organisation and the maintenance of differentiated secretory function J Cell Sci 1992 102: 361–372
Krill D et al. A simple method for the isolation and culture of epithelial and stromal cells from benign and neoplastic prostates Urology 1997 49: 981–988
Martin FL et al. Activation of genotoxins to DNA-damaging species in exfoliated breast milk cells Mutat Res 2000 470: 115–124
Kooiman GG et al. The influence of dietary and environmental factors on prostate cancer risk Prostate Cancer PD 2000 3: 256–258
Gleason DF . Histologic grading and staging of prostatic carcinoma In: Tannenbaum M (ed) Urology Pathology: The Prostate Lea & Febiger: Philadelphia 1977 pp 171–197
Grover PL, Martin FL . The initiation of breast and prostate cancer Carcinogenesis 2002 (in press)
Acknowledgements
This work was supported by the Association for International Cancer Research, the Cancer Research Campaign and the Department of Health. Francis L. Martin is currently supported by a grant from the North West Cancer Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, F., Cole, K., Muir, G. et al. Primary cultures of prostate cells and their ability to activate carcinogens. Prostate Cancer Prostatic Dis 5, 96–104 (2002). https://doi.org/10.1038/sj.pcan.4500579
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500579
Keywords
This article is cited by
-
Metabolism and biomarkers of heterocyclic aromatic amines in humans
Genes and Environment (2021)
-
Quantified gene expression levels for phase I/II metabolizing enzyme and estrogen receptor levels in benign prostate from cohorts designated as high-risk (UK) versus low-risk (India) for adenocarcinoma at this organ site: a preliminary study
Asian Journal of Andrology (2010)
-
Comet assay: a reliable tool for the assessment of DNA damage in different models
Cell Biology and Toxicology (2009)
-
Differential gene expression in the peripheral zone compared to the transition zone of the human prostate gland
Prostate Cancer and Prostatic Diseases (2008)
-
Highly elevated PSA and dietary PhIP intake in a prospective clinic-based study among African Americans
Prostate Cancer and Prostatic Diseases (2007)