Abstract
Background:
Loss or mutations of the BRCA1 gene are associated with increased risk of breast and ovarian cancers and with prostate cancer (PCa) aggressiveness. Previously, we identified GADD153 as a target of BRCA1 protein, which increases doxorubicin sensitivity in human p53 −/− PCa cells (PC3). Considering that p53 is a crucial target in cancer therapy, in this work we investigated p53 role in the regulation of transcription of GADD153.
Methods:
We performed reverse transcription quantitative PCR (RT-qPCR), western blot and luciferase assays to analyze GADD153 and/or BRCA1 expression in response to ultraviolet or doxorubicin exposure in PC3 p53 stable-transfected cells and LNCaP (p53+/+) cells. BRCA1 protein recruitment to GADD153 promoter was studied by chromatin immunoprecipitation-qPCR. To assess expression of BRCA1 and/or p53 target genes, we used a panel of stable-transfected PCa cell lines. We finally analyzed these genes in vivo using BRCA1-depleted PCa xenograft models.
Results:
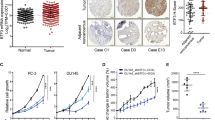
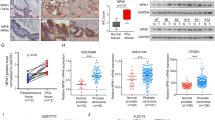
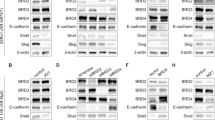
We found that GADD153 was highly induced by doxorubicin in PC3 cells; however, this response was totally abolished in LNCaP (p53wt) and in p53-restituted PC3 cells. Furthermore, BRCA1 protein associates to GADD153 promoter after DNA damage in the presence of p53. Additionally, we demonstrated that BRCA1 and/or p53 modulate genes involved in DNA damage and cell cycle regulation (cyclin D1, BLM, BRCA2, DDB2, p21WAF1/CIP1, H3F3B, GADD153, GADD45A, FEN1, CCNB2), EMT (E-cadherin, β-catenin, vimentin, fibronectin, slug, snail) and Hedgehog pathways (SHH, IHH, DHH, Gli1, PATCH1). Furthermore, xenograft studies demonstrated that BRCA1 knockdown in PC3 cells increased tumor growth and modulated these genes in vivo.
Conclusions:
Although BRCA1 induces GADD153 in a p53 independent manner, p53 abolished GADD153 induction in response to DNA damage. In addition, several important PCa targets are modulated by BRCA1 and p53. Altogether, these data might be important to understand the therapy response of PCa patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lowe SW, Ruley HE, Jacks T, Housman DE . p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 1993; 74: 957–967.
Blandino G, Levine AJ, Oren M . Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene 1999; 18: 477–485.
Olivier M, Hollstein M, Hainaut P . TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010; 2: a001008.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994; 266: 66–71.
Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE . Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994; 343: 692–695.
Douglas JA, Levin AM, Zuhlke KA, Ray AM, Johnson GR, Lange EM et al. Common variation in the BRCA1 gene and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2007; 16: 1510–1516.
Gallagher DJ, Gaudet MM, Pal P, Kirchhoff T, Balistreri L, Vora K et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res 2010; 16: 2115–2121.
Liu X, Holstege H, van der Gulden H, Treur-Mulder M, Zevenhoven J, Velds A et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci USA 2007; 104: 12111–12116.
Venkitaraman AR . Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002; 108: 171–182.
De Luca P, Vazquez ES, Moiola CP, Zalazar F, Cotignola J, Gueron G et al. BRCA1 loss induces GADD153-mediated doxorubicin resistance in prostate cancer. Mol Cancer Res 2011; 9: 1078–1090.
De Siervi A, De Luca P, Byun JS, Di LJ, Fufa T, Haggerty CM et al. Transcriptional autoregulation by BRCA1. Cancer Res 2010; 70: 532–542.
Smith JL, Freebern WJ, Collins I, De Siervi A, Montano I, Haggerty CM et al. Kinetic profiles of p300 occupancy in vivo predict common features of promoter structure and coactivator recruitment. Proc Natl Acad Sci USA 2004; 101: 11554–11559.
Xu CF, Chambers JA, Solomon E . Complex regulation of the BRCA1 gene. J Biol Chem 1997; 272: 20994–20997.
De Siervi A, De Luca P, Moiola C, Gueron G, Tongbai R, Chandramouli G et al. Identification of new Rel/NFkB regulatory networks by focused genome location analysis. Cell Cycle 2009; 8: 13.
MacLachlan TK, Somasundaram K, Sgagias M, Shifman Y, Muschel RJ, Cowan KH et al. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J Biol Chem 2000; 275: 2777–2785.
Anand S, Chakrabarti E, Kawamura H, Taylor CR, Maytin EV . Ultraviolet light (UVB and UVA) induces the damage-responsive transcription factor CHOP/gadd153 in murine and human epidermis: evidence for a mechanism specific to intact skin. J Invest Dermatol 2005; 125: 323–333.
Whibley C, Pharoah PD, Hollstein M . p53 polymorphisms: cancer implications. Nat Rev Cancer 2009; 9: 95–107.
Holstege H, Joosse SA, van Oostrom CT, Nederlof PM, de Vries A, Jonkers J . High incidence of protein-truncating TP53 mutations in BRCA1-related breast cancer. Cancer Res 2009; 69: 3625–3633.
Manie E, Vincent-Salomon A, Lehmann-Che J, Pierron G, Turpin E, Warcoin M et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res 2009; 69: 663–671.
Navone NM, Troncoso P, Pisters LL, Goodrow TL, Palmer JL, Nichols WW et al. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst 1993; 85: 1657–1669.
Moiola C, De Luca P, Cotignola J, Gardner K, Vazquez E, De Siervi A . Dynamic coregulatory complex containing BRCA1, E2F1 and CtIP controls ATM transcription. Cell Physiol Biochem 2012; 30: 596–608.
Agiostratidou G, Hulit J, Phillips GR, Hazan RB . Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia 2007; 12: 127–133.
Mitselou A, Batistatou A, Nakanishi Y, Hirohashi S, Vougiouklakis T, Charalabopoulos K . Comparison of the dysadherin and E-cadherin expression in primary lung cancer and metastatic sites. Histol Histopathol 2010; 25: 1257–1267.
Chao YL, Shepard CR, Wells A . Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 2010; 9: 179.
Hudson LG, Zeineldin R, Stack MS . Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis 2008; 25: 643–655.
Paredes J, Correia AL, Ribeiro AS, Milanezi F, Cameselle-Teijeiro J, Schmitt FC . Breast carcinomas that co-express E- and P-cadherin are associated with p120-catenin cytoplasmic localisation and poor patient survival. J Clin Pathol 2008; 61: 856–862.
Emadi Baygi M, Soheili ZS, Essmann F, Deezagi A, Engers R, Goering W et al. Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines. Tumour Biol 2010; 31: 297–307.
Epstein EH . Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 2008; 8: 743–754.
Chen M, Feuerstein MA, Levina E, Baghel PS, Carkner RD, Tanner MJ et al. Hedgehog/Gli supports androgen signaling in androgen deprived and androgen independent prostate cancer cells. Mol Cancer 2010; 9: 89.
Sanchez P, Clement V, Ruiz i Altaba A . Therapeutic targeting of the Hedgehog-GLI pathway in prostate cancer. Cancer Res 2005; 65: 2990–2992.
Zhang J, Lipinski R, Shaw A, Gipp J, Bushman W . Lack of demonstrable autocrine hedgehog signaling in human prostate cancer cell lines. J Urol 2007; 177: 1179–1185.
Acknowledgements
This research was supported by the Argentinean Agency of Science and Technology (ANPCyT PICT 2006-00228, PICT 2006-00367 and PICT 2010-00431). E. Vazquez and A. De Siervi are members of the career of scientific researcher at the National Research Council (CONICET). P De Luca holds postdoctoral fellowship from CONICET. C Moiola and F Zalazar hold PhD scholarships from CONICET. K. Gardner is a principal investigator at the NCI, NIH (USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
De Luca, P., Moiola, C., Zalazar, F. et al. BRCA1 and p53 regulate critical prostate cancer pathways. Prostate Cancer Prostatic Dis 16, 233–238 (2013). https://doi.org/10.1038/pcan.2013.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2013.12
Keywords
This article is cited by
-
Gene expression profiling of homologous recombination repair pathway indicates susceptibility for olaparib treatment in malignant pleural mesothelioma in vitro
BMC Cancer (2019)
-
4C-seq revealed long-range interactions of a functional enhancer at the 8q24 prostate cancer risk locus
Scientific Reports (2016)
-
Prediction and Characterisation of the System Effects of Aristolochic Acid: A Novel Joint Network Analysis towards Therapeutic and Toxicological Mechanisms
Scientific Reports (2015)
-
BRCA1 gene variant p.P142H associated with male breast cancer: a two-generation genealogic study and literature review
Familial Cancer (2015)