Abstract
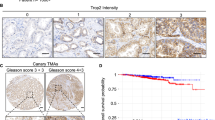
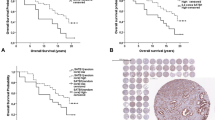
Syndecans are a four-member family of transmembrane heparan sulphate proteoglycans that have different functions in cell signalling, adhesion, cytoskeleton organization, migration, proliferation, and angiogenesis. Several studies investigated the role of syndecan-2 (SDC2) in different carcinomas; however, only one being focused on SDC2 in prostate cancer. SDC2 expression and relationship with established prognostic features were assessed in a cohort of 86 patients treated with radical prostatectomy for clinically localized prostate adenocarcinoma. SDC2 expression was present in the majority of prostate cancers and absent in only 11.6% of cases. SDC2 expression was also recorded in cells of prostatic intraepithelial neoplasia, whereas normal prostatic epithelial tissue and stroma did not express SDC2. SDC2 overexpression in prostate cancer was significantly associated with established features indicative of worse prognosis such as higher preoperative PSA (P=0.011), higher Gleason score (P<0.001), positive surgical margins (P<0.003), and extraprostatic extension of disease (P<0.003). Moreover, expression of SDC2 was also associated with biochemical disease progression on univariate analysis (P<0.001). Study results supported the potential role of SDC2 in prostatic carcinogenesis and cancer progression. Moreover, SDC2 could serve as an additional prognostic marker that might help in further stratifying the risk of disease progression in patients with prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rapraeger AC . Syndecan-regulated receptor signaling. J Cell Biol 2000; 149: 995–998.
Essner JJ, Chen E, Ekker SC . Syndecan-2. Int J Biochem Cell Biol 2006; 38: 152–156.
Simons M, Horovitz A . Syndecan-4-mediated signaling. Cell Signal 2001; 13: 855–866.
Woods A, Couchman JR . Syndecan-4 and focal adhesion function. Curr Opin Cell Biol 2001; 13: 578–583.
Choi S, Lee E, Kwon S, Park H, Yi JY, Kim S et al. Transmembrane domain-induced oligomerization is crucial for the functions of syndecan-2 and syndecan-4. J Biol Chem 2005; 280: 42573–42579.
Noguer O, Villena J, Lorita J, Vilaro S, Reina M . Syndecan-2 downregulation impairs angiogenesis in human microvascular endothelial cells. Exp Cell Res 2009; 315: 795–808.
Marynen P, Zhang J, Cassiman JJ, Van den Berghe H, David G . Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem 1989; 264: 7017–7024.
Tkachenko E, Rhodes JM, Simons M . Syndecans: new kids on the signaling block. Circ Res 2005; 96: 488–500.
Han I, Park H, Oh ES . New insights into syndecan-2 expression and tumourigenic activity in colon carcinoma cells. J Mol Histol 2004; 35: 319–326.
Huang JW, Chen CL, Chuang NN . P120-GAP associated with syndecan-2 to function as an active switch signal for Src upon transformation with oncogenic ras. Biochem Biophys Res Commun 2005; 329: 855–862.
Kim Y, Park H, Lim Y, Han I, Kwon HJ, Woods A et al. Decreased syndecan-2 expression correlates with trichostatin-A-induced morphological changes and reduced tumorigenic activity in colon carcinoma cells. Oncogene 2003; 22: 826–830.
Choi S, Kim Y, Park H, Han IO, Chung E, Lee SY et al. Syndecan-2 overexpression regulates adhesion and migration through cooperation with integrin alpha2. Biochem Biophys Res Commun 2009; 384: 231–235.
Gulyas M, Hjerpe A . Proteoglycans and WT1 as markers for distinguishing adenocarcinoma, epithelioid mesothelioma, and benign mesothelium. J Pathol 2003; 199: 479–487.
Park H, Kim Y, Lim Y, Han I, Oh ES . Syndecan-2 mediates adhesion and proliferation of colon carcinoma cells. J Biol Chem 2002; 277: 29730–29736.
Huang X, Xiao DW, Xu LY, Zhong HJ, Liao LD, Xie ZF et al. Prognostic significance of altered expression of SDC2 and CYR61 in esophageal squamous cell carcinoma. Oncol Rep 2009; 21: 1123–1239.
Contreras HR, Ledezma RA, Vergara J, Cifuentes F, Barra C, Cabello P et al. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial-mesenchymal transition markers, E-cadherin and beta-catenin, in prostate cancer. Urol Oncol 2009. May 16. [Epub ahead of print], PMID:19450993, doi:10.1016/j.urolonc.2009.03.018.
Sobin LH, Wittekind C (eds) International Union Against Cancer (UICC): TNM Classification of Malignant Tumours. 5th edn. Wiley Liss: New York, USA, 1997. pp 170–173.
Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, Gasser TC et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate 2003; 55: 20–29.
Shariat SF, Svatek RS, Kabbani W, Walz J, Lotan Y, Karakiewicz PI et al. Prognostic value of syndecan-1 expression in patients treated with radical prostatectomy. BJU Int 2008; 101: 232–237.
Kiviniemi J, Kallajoki M, Kujala I, Matikainen MT, Alanen K, Jalkanen M et al. Altered expression of syndecan-1 in prostate cancer. APMIS 2004; 112: 89–97.
Chen D, Adenekan B, Chen L, Vaughan ED, Gerald W, Feng Z et al. Syndecan-1 expression in locally invasive and metastatic prostate cancer. Urology 2004; 63: 402–407.
Shimada K, Nakamura M, De Velasco MA, Tanaka M, Ouji Y, Konishi N . Syndecan-1, a new target molecule involved in progression of androgen-independent prostate cancer. Cancer Sci 2009. April 21. [Epub ahead of print], PMID:19432893, doi:10.1111/j.1349-7006.2009.01174.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popović, A., Demirović, A., Spajić, B. et al. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis 13, 78–82 (2010). https://doi.org/10.1038/pcan.2009.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2009.43
Keywords
This article is cited by
-
The inhibitory effect of betulinic acid on epithelial–mesenchymal transition pathway in renal cell carcinoma
Medical Oncology (2022)
-
Proteoglycan-based diversification of disease outcome in head and neck cancer patients identifies NG2/CSPG4 and syndecan-2 as unique relapse and overall survival predicting factors
BMC Cancer (2015)
-
RKIP and HMGA2 regulate breast tumor survival and metastasis through lysyl oxidase and syndecan-2
Oncogene (2014)
-
Altered expression patterns of syndecan-1 and -2 predict biochemical recurrence in prostate cancer
Asian Journal of Andrology (2011)